Prof. Kamakaka`s Lecture 1 Notes
advertisement

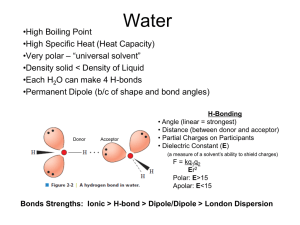
Water and pH What is Biochemistry? Water is the medium for life on earth What are the molecules in living cells? Macromolecules All macromolecules comprise multiple carbons joined together in some way. Two important properties of macromolecules: Polymers are made from monomeric units Amino acids-------> Nucleotides-------> Sugars---------> Fatty acids--------> Condensation and Hydrolysis Two types of reactions affect bonds- condensation and hydrolysis If you have two monomers. One has a hydroxyl group and the other has a hydrogen group. And these two react with one another and, with the use of energy, water is released (dehydration), and the two monomers are joined. The converse of that is hydrolysis where you add water and use energy to break up a polymer and release a monomer. Interactions Electronegativity= Valance= Integral to understanding macromolecules is the notion of chemical bonds To convert kilocalories into kilojoules multiply by 4.184 Covalent and Ionic Bonds Covalent (100-400 kcal/mol) H Cl attraction Na Ionic (100-300 kcal/mol) Cl repulsion Hydrogen and hydrophobic interactions H2 (12-16 kcal/mol) attraction between a hydrogen atom in one molecule and a atom of high electronegativity in another molecule. It is an intermolecular force, not an intramolecular force. Hydrophobic Interactions To convert kilocalories into kilojoules multiply by 4.184 Van der Waals Polar and ionic molecules have positive and Nonpolar molecules are hydrophobic (means "water fearing"). They do not dissolve in water. negative charges and are therefore attracted to water molecules because water molecules are also polar. They are said to be hydrophilic because they interact with (dissolve in) water by forming hydrogen bonds. Structure of Water Structure of Water Hydrogen bonds in water Common Hydrogen bonds Important hydrogen bonds Strong and weak hydrogen bonds Polar, Non-polar and amphipathic molecules Water and NaCl- water as a solvent Water cages and amphipathic molecules Water and Lipid micelles Enzyme-substrate interactions and water Colligative properties Some properties of a solution do not depend on the chemical properties of the dissolved substance but does depend upon the NUMBER of solute molecules in a unit of water melting point boiling point osmolarity Osmotic pressure and water in cells Water moves from high water conc to low water conc. A solute separated from water by a semi-permeable membrane. Osmosis Water and cell membranes xxxxxxx Ionization of water H20 Kw (equilibrium constant) Kw= [H+][OH-] [H2O] Kw= [H+][OH-] [55.5] Measured Kw= 1.8x10-16M [1.8x10-16][55.5]= 1x10-14= [H+][OH-] H+ + OH- Concentration of water Weight of 1 liter of water at 1 atmosphere is Molecular weight of water in g is Therefore concentration of water is Water spontaneously dissociates into H+ and OH(Water molecules can function as both acids and bases) H2O is in equilibrium with H+ and OHThe dissociation constant for water is 1.8x10-16 Concentration of H+ in water is pH is negative logarithm (base 10) of the molar concentration of hydrogen ions (H+) i.e. pH of water is 7 at a particular temperature (~25C) pH scale Low pH= High pH= pH+pOH=14 pH Scale An Acid gives rise to excess of H+ in aqueous solution - a proton donor is defined as an acid Strong acids and bases - are completely ionized in water in pH range 0-14 Weak acids and bases - defined as incompletely ionized in water in pH range 0-14 Ka is a value used to describe the tendency of compounds to dissociate (the dissociation constant) HA----> H + A (A is the conjugate base of the acid HA) Ka= Ka =equilibrium constant Ka is the dissociation value for a compound in water; A stronger acid dissociates completely and will have a large [H+] concentration and hence a large Ka. Due to the many orders of magnitude spanned by Ka values, a logarithmic measure of the constant is more commonly used The value of the pKa changes with temperature. pKa values are temperature dependent in a non-linear way. Acid dissociation Acetic acid Ka= 1.74x10-5 M pKa = 4.76 HCl Ka= pKa= -4 Ka = dissociation constant pKa = (strong acid----->large Ka----->small pKa) (pKa measures acidity) An acid is a substance that dissociates in aqueous solution, releasing H+ (a proton) The liberated proton combines with a water molecule to give a hydronium HA(acid) + H2O(base) <----> A-(conjugate base) + H3O+(conjugate acid) The designation of an acid or base as "conjugate" depends on the context. H2O (acid)+B (Base) <---- OH- (conjugate base)+ BH+ (conjugate acid) Acids and bases are thus regarded simply as donors and acceptors of protons respectively. You can measure pKa of an acid by performing a titration curve. Take and acid, measure pH--- say 1.0 Add a strong base such as NaOH and after each addition of NaOH, you measure pH The pH at mid-point of the titration is the pKa for that acid at that temperature pH=pKa=50% ionized acid It is possible to calculate the equilibrium concentration of acids and bases in solution when the pH is known. pH=pKa+log[A-]/[HA] Titration of an acid Buffers Buffers are mixtures of weak acids and their anions • Buffers resist change in pH • At pH = pKa, there is a 50:50 mixture of acid and anion forms of the compound • Buffering capacity of acid/anion system is greatest at pH = pKa O CH3C O CH3C OH H+ O- Buffers Weak acids or weak bases function as buffers because they do not fully dissociate They end up being a mixture of weak acid and its salt Weak acids (HA) are a reserve of protons that can neutralize any OH- added into the reaction The salt of a weak acid (A-) acts as a base and can neutralize any H+ added into the reaction Buffers can both bind or release protons and in so doing prevents the pH from changing rapidly. The pH that a buffer can maintain varies and is determined by its pKa A buffer is at optimal strength when there is equal amount of HA and A. This occurs when pH=pKa Buffers Acetic acid + sodium acetate buffer (pKa=4.76) CH3COOH ---> CH3COO- + H+ <------------ Since this is a weak acid, very little dissociates and most of the CH3COOH is undissociated. A buffer is a mixture of a weak acid (CH3COOH) and its conjugate base (CH3COONa) dissolved in water. (Water and sodium ions which are present aren't important to the argument). Lets add acid (H+) to this buffer. Buffers CH3COOH ---> CH3COO- + H+ <------------ Now lets add a base (OH-) to this buffer. This time the situation is a bit more complicated because there are two processes which can remove hydroxide ions. Scenario1 Scenario2 Acetate has a pKa of 4.76. It is a good buffer around 4.76 Buffers and water Acetic acid + acetate buffer: absorbs H+ or OH- Henderson Hasselbalch Equation It describes titration curves by relating pH, pKa and buffer concentration pH = pKa + log [A-] [HA] Useful for biochemists: 1) You can calculate pKa from pH and concentration of proton acceptor and donor 2) You can calculate pH from pKa and concentration of proton acceptor and donor 3) You can calculate the ratio of proton donor and acceptor based on pH and pKa Acid-base pairs Acetic acid Ka= 1.74x10-5 M pKa = 4.76 O O CH3C CH3C H+ OH Ammonium (urine) Ka=5.62x10-10 pKa= 9.25 NH3+ NH4+ O CH2 O- CH2 C Glycine-Carboxyl Ka= 4.57x10-3 M pKa= 2.34 O H+ C O- OH NH3+ Glycine-Amino Ka= 2.51x10-10 M pKa= 9.60 H+ NH3 NH3+ (Also phosphate in cytosol Bicarbonate in blood) CH2 O NH2 CH2 C O- O H+ C O- Titration curves Amino acid titration curves Histidine side chain is a weak acid







![[H 2 PO 4 - ] = 0.800 M.](http://s2.studylib.net/store/data/005623813_1-92875a3e2acb84ddbb79ead23a1c6630-300x300.png)