Chemistry 125: Lecture 45
January 28, 2011
This
Nucleophilic Substitution
and Mechanistic Tools:
Solvent, Leaving Group
PET Scanning
Pentavalent Carbon?
For copyright
notice see final
page of this file
SN2 Nucleophilic Substitution
Solvent
Nu:
R-L
Nucleophile
Substrate
(+)
(-)
Nu-R
L
Product
Leaving
Group
the Pragmatic Logic
of Proving a Mechanism
with Experiment & Theory
(mostly by disproving all
alternative mechanisms)
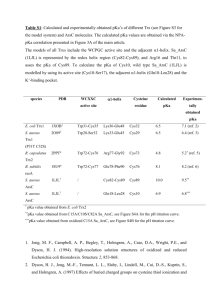
Rate Constant Dependance on
Nucleophile
Nu
R-L
Leaving
Substrate Group
krel
pKa
[1]
-1.7
F-
80
3.2
Cl-
1,000
-8
Br-
10,000
-9
HO-
16,000
15.7
I-
80,000
-10
HS-
126,000
7
Nu-R
L
Polar solvents accelerate reactions
that generate (or concentrate) charge,
and vice versa.
(NuH+)
krel
CH3I
in H2O
harder [1]
to break 14
H-bonds
to smaller
ions 160
krel
CH3Br
in Acetone
Backwards
H2O
(-)
(+)
11
5
Sensible
Nu:
Solvent
[1]
e.g. J&F Sec. 7.4dg
Rate Constant Dependance on
Nu:
Nucleophile
Solvent
R-L
Leaving
Substrate Group
L
(+)
Nu-R
(-)
L
pKa (LH+)
HO-
v. bad
15.7
HS-
bad
7
FH2O
bad
good
3.2
-1.7
RSO2O-
good
-3
Cl-
good
-8
BrI-
good
v. good
-9
-10
Weak bases are
good leaving groups
(Stable anions form easily.
Those that don’t hold
tightly to H+ don’t hold
tightly to Nud- C in the
NudC
LdSN2 transition state,
as expected)
e.g. J&F Sec. 7.4e
How?
With permission of the Edmund S. Muskie Archives
and Special Collections Library – Bates College
Molecule specifically
designed and prepared
to test these
mechanistic questions
Cl
Lawrence H. Knox
Paul D. Bartlett
(1908-1964)
(1907-1997)
Bartlett and Knox *
(J.Am.Chem.Soc. - 1939)
Need a Fabulous
Leaving Group!
O
Cl
now an “allylic”
N
H + Nrearrangement shifts
H2O
HO
H
O 2 H from N to O
N
pKa
+
Cl
H
+ NH2 H-NH2 = 34
Can generate even +
H-N+H3 = 9
bridgehead cation! +
Near the end of the semester we’ll discuss
R-COOH
R-CNH2
R-NH2
How?
Molecule specifically
designed and prepared
to test these
mechanistic questions
Cl
Bartlett and Knox *
(J.Am.Chem.Soc. - 1939)
Rate Constant Dependance on
Nu:
Nucleophile
Solvent
R-L
Leaving
Substrate Group
L
(-)
(+)
Nu-R
L
pKa (LH+)
HO-
v. bad
15.7
HS-
bad
7
FH2O
bad
good
3.2
-1.7
RSO2O-
good
-3
Cl-
good
-8
BrI-
good
v. good
-9
-10
Weak bases are
good leaving groups
(H like R, as expected)
R-OH
v. bad
R-OH2+
good
(acid catalysis)
R-OSO2R’ good
(Kenyon/Phillips)
e.g. J&F Sec. 7.4e
OH Leaving-Group-Trick Lore (e.g. J&F sec 7.4f)
OH2+
BrCH3-CH2-OH
Br
CH3-CH2 + OH-
Bad leaving group
Br
CH3-CH2 + OH2
H-O+H2 pKa -1.7
Good leaving group
H-OH pKa 16
H-Br pKa -5
Br-
CH3-CH2-O+H2
Ether Cleavage by HBr
Br-
excess 47% HBr
O
Br
8 hr
Br
+
O
Good Leaving Group
OH
H
Br
Good
Nucleophile
OH Leaving-Group-Trick Lore (e.g. J&F sec 7.4f)
OSO2R
O H
PhCH2 CH
CH3
Cl SO2
H OSO2
CH3
CH3
toluenesulfonic acid
pKa -3
Kenyon & Phillips (1923)
O SO2
PhCH2 CH
CH3
CH3
“tosylate”
O
O C CH3
O
O C CH3
PhCH2 CH
CH3
OH Leaving-Group-Trick Lore (e.g. J&F sec 7.4f)
OSOCl
b.p.
75°C
61°C
gases
OH Leaving-Group-Trick Lore (e.g. J&F sec 7.4f)
OPXn
(CH3)2CHCH2OH
PBr3
(CH3)2CHCH2Br + P(OH)3
(58%)
-10°C, 4 hr
Larger rings allow flattening
Inaccessible for
SN2
of bridgehead
cation.
PCl5
+ OH
PClx
CaCO3
ether
0°C, 3 min
Substitution of RO- for Cl- at P
(probably A/D mechanism)
generates good leaving group.
Cl
(“100%”)
OH Leaving-Group-Trick Lore (e.g. J&F sec 7.4f)
Appel Reaction
+
OP Ph3
(Wikipedia)
pKa ~17
Substitution
of P for
CCl3 at Cl
pKa 24
A/D
substitution
at P
(vacant d-orbitals)
H
e.g.
D
D
OH
Cl
f3P
CCl4
25°C, 24 hr
H
D
D
(~85%)
Using SN2 Mechanistic Knowledge to
Maximize Synthetic Speed for PET scanning
(from Loudon, Org. Chem.)
http://en.wikipedia.org/wiki/Positron_emission_tomography
Connecting
simultaneous
scintillations shows
where 18F’s were.
18O= + 7 MeV proton
- neutron
18F-
or
11C t ~ 20 min
1/2
13N t ~ 10 min
1/2
15O t ~ 2 min
1/2
t1/2
110 min
+e
positron
+18O=
proton
neutron
Need to get 18F
where tumor is
and you have to do so
within a few hours of
preparing the element.
Yale PET
What to
synthesize?
Protected Triflate to 2-Fluoroglucose - ASAP
Glucose
2-Fluoroglucose
KF18
SN2
?
F18
and by K+cation
would
suck up
18F Maybe
tied upitby
H-bonding
2-Fluoroglucose as well.
HO a horrid leaving group
trifluormethanesulfonate
wrong C-OH could be attacked
(Triflate)
“protection” is
Now to introduce 18F
sugar chemistrySN2 inversion gives wrong configuration
start with
Rapid metabolism ofMannose
tumor
sucks up glucose.
SN2 Problems :
This kind of
well known in
pKa ~ -14
Cl-SO2CF3
O
AcO = CH3C-O-
(acetate protecting group)
Protected Triflate to 2-Fluoroglucose - ASAP
Glucose
2-Fluoroglucose
KF18
SN2
“deprotection” H2O H+
and by K+cation
18F tied up by H-bonding
K+ K+ 18FCH3CN
(aprotic solvent)
Cl-SO2CF3
Vertical Section
viewed from front
PET Scan Image
measured 1 hour
after administering
fludeoxyglucose (18F)
shows high glucose
metabolism in brain
and in a cancerous
lymph node.
Horizontal Section
viewed from beneath
Linus Pauling
1901-1994
Akira Kouchiyama
Tools for Testing
(i.e. Excluding) Mechanisms:
Stereochemistry
Rate Law
Rate Constant
Structure
X-Ray and Quantum Mechanics
So far we’ve just been beating up
on the D/A mechanism
(trivalent C intermediate)
though there are cases (SN1)
where it in fact applies.
The tougher problem is to distinguish between concerted
and A/D with a very weakly stabilized intermediate.
(see supplementary reading on Course website)
Might there be Pentavalent A/D Intermediate
instead of a Concerted SN2 Transition State?
Nu
C
L
Transition
State
Nu
C
L
Pentavalent
Intermediate
Might there be Pentavalent A/D Intermediate
instead of a Concerted SN2 Transition State?
2.64 Å
2.64 Å
Quantum Mechanics
says Transition State
for H2O attacking
protonated t-BuOH.
1.88 Å
1.88 Å
Quantum Mechanics
says Transition State
for OH- attacking
less crowded CH3OH.
But neither reaction is practical in the laboratory!
What does experiment say?
X-ray?
End of Lecture 45
Jan. 28, 2011
Copyright © J. M. McBride 2011. Some rights reserved. Except for cited third-party materials, and those used by visiting
speakers, all content is licensed under a Creative Commons License (Attribution-NonCommercial-ShareAlike 3.0).
Use of this content constitutes your acceptance of the noted license and the terms and conditions of use.
Materials from Wikimedia Commons are denoted by the symbol
.
Third party materials may be subject to additional intellectual property notices, information, or restrictions.
The following attribution may be used when reusing material that is not identified as third-party content:
J. M. McBride, Chem 125. License: Creative Commons BY-NC-SA 3.0