Chemistry study guide with answers
advertisement

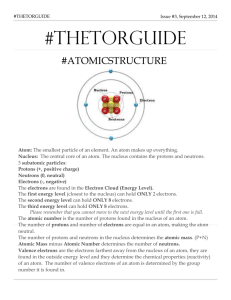
Chem Test Study Guide Know the Law of Conservation of Matter states that ___matter is neither created nor destroyed______ during a chemical reaction. Remember that because of the Law of conservation of matter, the total __mass____________ of the _________reactants_________________ must equal the mass of the __________products__________. Know the three subatomic particles, their location in the atom, and their charges. Protons (+) nucleus neutrons (0) nucleus Electrons (-) electron cloud Remember that ____protons_______________ and _________neutrons________________________ have most of the mass in an atom. The atomic number of an atom tells me: number of protons and number of electrons The number of ______protons________________________ is unique to each element. A _____group_____________ is the column on the periodic table and a _____period__________________ is a row. The _______atomic mass____________ of an atom is the weighted average mass of all an atom’s isotopes. The most reactive nonmetals are ______group 17: the halogens________________________ The _______most reactive_________________________ elements on the periodic table have one or seven valence electrons. The number of valence electrons _____increase________________________ as you move from left to right on the periodic table. Group 16 elements have ____4 more_____________ valence electrons than group 2. An element’s tendency to react is closely related to its ____valence electrons_______________. Members of the nitrogen family have ____5______ valence electrons. Members of the halogens (group 17) are not anything like this group: ___noble gases_________________________. Members of the Noble gases (group 18) have a stable ________outer energy level/ valence _______________ configuration. Atoms gain or lose electrons to achieve a ____stable or complete outer energy level_______________________________________________. In an electron dot diagram, the symbol for the element is used to represent the __nucleus__________________. Formation of _____ionic_________________ bonds involves the transfer of ______valence electrons____________________. The substance with the formula KI contains one atom of _____potassium (K)________ and one atom of __Iodine (I)_________. A subscript in a formula indicates the ______number of atoms of a certain element in a compound________________. What type of bond forms molecules? ___covalent_________________________ These compounds are composed of ___nonmetals______________________. The formula for the ionic compound beryllium chloride is ___BeCl2________________________ because there are 2 chlorine atoms for every one beryllium atom. In the name carbon dioxide, the prefix of the second word indicates there are ___2 oxygen atoms____________________________. The substances that undergo a change in a chemical reaction are called _____reactants_____________________. Hydrochloric acid, HCl, is added to solid NaOH. After the reaction is complete, you are left with NaCl dissolved in water. What are the products in this reaction? H2O (water) and NaCl (salt) What does the arrow mean in a chemical equation? Yields/ makes/ produces/ equals In a chemical reaction, an iron atom became the ion Fe2+. What happened to the iron atom? Lost 2 electrons The total amount of energy before and after a chemical reaction is the same. Thus energy is _____conserved_______________________ during a chemical reaction. If the temperature of a chemical reaction is increased, then the reaction time will ____increases________________. In a polymer, monomers are linked by ___covalent bonds_________________________ bonds. Rubber is both an artificial and natural ____polymer______________________.