Electrons - Cloudfront.net
advertisement
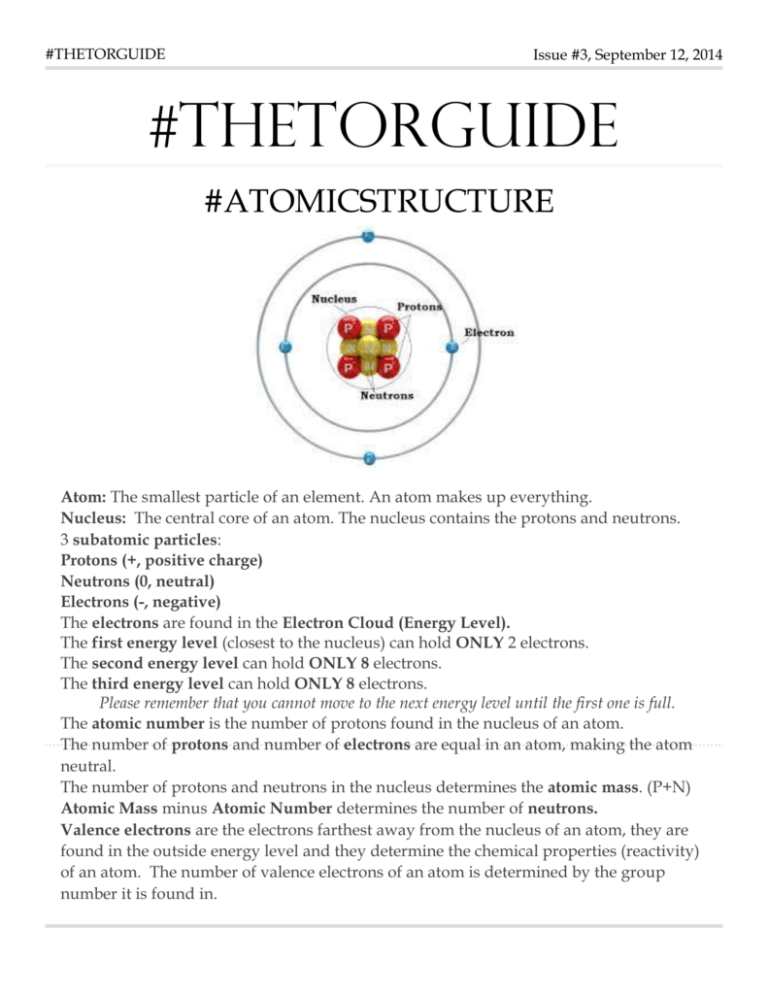
#THETORGUIDE Issue #3, September 12, 2014 #THETORGUIDE #ATOMICSTRUCTURE Atom: The smallest particle of an element. An atom makes up everything. Nucleus: The central core of an atom. The nucleus contains the protons and neutrons. 3 subatomic particles: Protons (+, positive charge) Neutrons (0, neutral) Electrons (-, negative) The electrons are found in the Electron Cloud (Energy Level). The first energy level (closest to the nucleus) can hold ONLY 2 electrons. The second energy level can hold ONLY 8 electrons. The third energy level can hold ONLY 8 electrons. Please remember that you cannot move to the next energy level until the first one is full. The atomic number is the number of protons found in the nucleus of an atom. The number of protons and number of electrons are equal in an atom, making the atom neutral. The number of protons and neutrons in the nucleus determines the atomic mass. (P+N) Atomic Mass minus Atomic Number determines the number of neutrons. Valence electrons are the electrons farthest away from the nucleus of an atom, they are found in the outside energy level and they determine the chemical properties (reactivity) of an atom. The number of valence electrons of an atom is determined by the group number it is found in. Reactivity Increases. (**Remember the more valence electrons, the more stable you become and less reactive you are.) Reactivity decreases down a group Most Valuable Scientist Extra Credit! Due Friday, September 19 Design your name using the elements from the Periodic Table!! Each element used should contain the atomic number, chemical symbol, name of the element and atomic mass. It needs to be unique and colorful. Use whatever materials you have around and have fun!! I want to tell you all Thank You! Thank you for making such a huge impact on me everyday. You may not realize it now, but you are so important! You all are going to rock this 3 week assessment!!! Thank you for your patience and determination. -MRS. S ELIJIO MORENO!!!!!!!











