Midterm Jeopardy Review Questions
advertisement
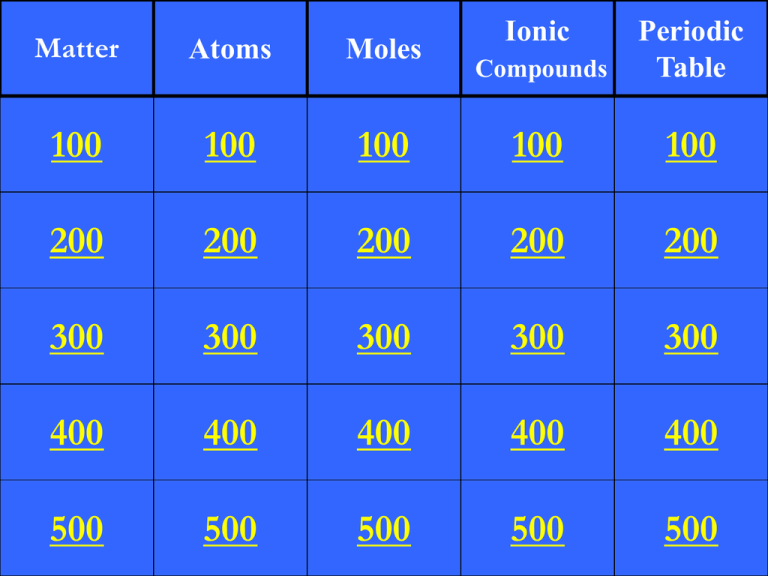
Compounds Periodic Table 100 100 100 200 200 200 200 300 300 300 300 300 400 400 400 400 400 500 500 500 500 500 Matter Atoms Moles 100 100 200 Ionic 100 A homogeneous mixture of metals. What is an alloy? 200 Which of the following is not considered matter? a. b. c. d. cells radiation water smoke What is b, radiation? 300 Give an example of a heterogeneous mixture. What is ______? 400 Which temperature on the graph shows the melting point? What is 0°C? 500 Electricity can convert oxygen (O2) into ozone (O3). The ozone created by this process is a(n) _____ of oxygen. What is an allotrope? 100 Quantum numbers describe the locations of protons in an atom. True or false? What is false? 200 Name given to electrons in the outer shell that determine properties of atoms. What is valence? 300 Atoms of the same element with the same number of protons but different numbers of neutrons are called _____. What is isotopes? 400 Electrons at low energy is called the ______ state. What is ground? 500 The model of the atom that is accepted to be true today. What is quantum? 100 This person is credited with determining the value of the mole. Who is Avogadro? 200 Number of particles in 1 mole. What is 6.022 x 23 10 ? 300 Units for molar mass. What is g/mol? 400 Number of grams in 1.4mol of Be. What is 13? 500 Number of particles in 10.0g of Br. What is 7.54 x 22 10 ? 100 An atom or group of atoms that has a charge? What is an ion? 200 Name given to atoms that lose electrons. What is cation? 300 Ionic compounds, as a whole, have ____ charge. What is no/neutral? 400 Name the following compound: Mg(NO3)2. What is magnesium nitrate? 500 Write the formula for the following compound: copper (II) sulfate. What is CuSO4. 100 This person developed the first periodic table and ordered elements by atomic mass. Who is Mendeleev? 200 Elements with similar properties are placed in _____ on the periodic table. What are groups? 300 The trend seen in atomic radius as you move down a group. What is increases? 400 The most reactive metals are found in which group number on the periodic table? What is group 1? 500 Place the following elements in order of decreasing ionization energy: Cl, F, Br. What is F, Cl, Br? Final Jeopardy Question! A metal has a specific heat of 0.335J/gK. The metal has a mass of 10.g, absorbs 4.09J of heat, and has an initial temperature of 200.K. What is the final temperature of the metal? CP = q/(mT) Answer: 201K