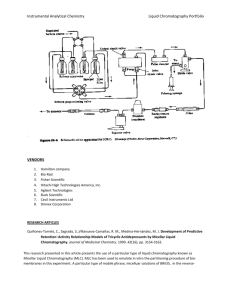
Chapter 38 - Chromatography and Instrumentation
advertisement

Chapter 38 - Chromatography and Instrumentation Section A – Introduction to Chromatography Chromatography *Mobile Phase – gas or liquid that carries the parts of the sample **Stationary Phase – the part of the apparatus that does not ______________ with the sample Uses of Chromatography Identify – determine the identity of a mixture or components based on known components example – to identify the mixture found in a ________________ at a crime scene. Purify – separate components in order to _______________ one of interest for further study _______________– determine the amount of the a mixture and/or the components present in the sample. Example – drugs in urine or blood samples Section B – Types of Chromatography 1. Paper Chromatography How this method works : In paper chromatography the mixture is dotted onto the bottom of _____________ paper. The paper is dipped into a _______________, which will rise up the paper and carry the mixture with it. Substances that are more soluble in the solvent will be carried quickly and substances that are less soluble will be carried slowly. In this way the mixture is separated. Stationary phase = Mobile phase = 2. Thin Layer Chromatography TLC This method works in a similar way to paper chromatography but is normally quicker and gives better separations Stationary phase = Mobile phase = Use in separating mixture found in ________________ St.Dominic’s College Chemistry notes Page 1 Chapter 38 - Chromatography and Instrumentation 3. Column Chromatography This method works in a similar way to paper chromatography but the mixture and the solvent are added to the top of the column and move downwards during the process. Stationary phase = Mobile phase = Use: 4. High Performance Liquid Chromatography (HPLC) This works in a similar way to column chromatography but is more sophisticated - Separates parts of a mixture which are very similar to each other. Better detection methods can be used - highly automated and extremely sensitive. Mobile phase = Solvent of high ________________. Instead of a solvent being allowed to drip through a column under gravity, it is forced through under high __________ - much faster. Stationary phase = Solid particles - ____________ particle size which gives a much greater _____________ area for interactions between the stationary phase and the molecules flowing past it. This allows a much better separation of the components of the mixture. Used for measuring levels of growth promoters and vitamins in meat St.Dominic’s College Chemistry notes Page 2 Chapter 38 - Chromatography and Instrumentation 5. Gas Chromatography How this method works 1. The injector for the sample is contained in an oven. The sample ____________ and is carried into the column as a gas by the ___________ gas. This method is most suitable for a mixture of volatile substances. 2. The coiled column is packed with finely ground ________ coated with non-volatile _________. The column is _____________ than the injector oven 3. Different parts of the mixture: May remain in the ___________- phase. May condense on the ______________ phase. May _________________ in the liquid on the surface of the stationary phase. 4. Separation happens because different components will have different retention times – based on boiling points and solubility in the liquid on the column. *Retention time means Stationary phase = Mobile phase = Used for measuring 1. Drug/ alcohol levels in blood samples 2. Measuring levels of pesticides in rivers St.Dominic’s College Chemistry notes Page 3 Chapter 38 - Chromatography and Instrumentation Section C – Mandatory experiment: Separation of a mixture of dyes using paper chromatography In this experiment a sample of ink (which is made of a mixture of dyes) is separated by paper chromatography. Procedure 1. Add solvent to the bottom of the tank to a depth of about 1cm. Cover the tank, and allow to stand for a few hours *This will allow the tank to become saturated with solvent vapour. 2. Using a pencil draw a line about ___________ up from the bottom of a piece of chromatography paper. *as pencil does not contain ink this line will not contaminate the other samples 3. Small dots of sample inks are put on the line on the paper. A capillary tube may be used if needed. Dry with a hairdryer and then repeat to get a concentrated dot of the sample. 4. Water is placed in the bottom of the chromatography jar – be sure that the water level is below the pencil line! *If the ink is below the water level it will dissolve into the water in the jar and will not be separated properly 5. Remove and dry. 6. Calculate and record the Rf values of each indicator: Distance travelled by the component Distance travelled by the solvent front *Rf values may be used to tentatively identify the components of a mixture of indicators. St.Dominic’s College Chemistry notes Page 4 Chapter 38 - Chromatography and Instrumentation Section D – Spectrometry 1. Mass spectrometry Based on the principle that _____________________ in a sample are _____________ based on their ___________________ moving in a ________ field. 5 main processes: 1. Vaporisation 2. Ionisation – atoms or molecules turned into _______ _______by bombarding them with electrons 3. Acceleration – positive ions passes through _____________ charged plates which accelerates the 4. Separation – positive ions pass through a magnetic field. Lighter ions are deflected more than ____________ ones so separation depends on ______________ 5. Detection – a detector responds to the ions as they fall on it. It uses the information to produce a mass spectrum for the sample. Use – 1. Measuring relative atomic masses 2. Determining the concentrations of ______________samples in a urine sample 2. Infra red spectroscopy (IR) This method works by molecules absorbing Infrared energy waves which cause particular bonds to ______________ and ______________ if present. It allows the identification of functional groups in organic or inorganic compounds ( for example a C=O bond would absorb IR energy at specific wavelengths not absorbed by other types of functional groups like O-H bonds). It is known as a “fingerprinting technique” and is a __________________ method but not a quantitative one. Use: To analyse which illegal drug is present in a sample 3. Ultraviolet spectroscopy (UV) This method works on the principle that molecules absorb ultraviolet energy waves, and that different types of molecules will absorb particular frequencies of uv light. The amount of energy absorbed will be ________________ proportional to the amount of substance present (even for colourless molecules) . It is a ________________ method. Use : analysing the amount of drugs in a medicine St.Dominic’s College Chemistry notes Page 5 Chapter 38 - Chromatography and Instrumentation Where is your learning at: Chromatography and Instrumentation Green : I know it all Orange : I have some idea – study the sections in more detail Red : I need to start studying this section CAN YOU Green Orange Red Define Chromatography ( or describe the principle by which it works) Describe how simple methods of Chromatography work – like paper Chromatography, Thin Layer Chromatography and Column Chromatography Describe an application of Thin layer Chromatography Hl - Describe how HPLC works and give an application HL - Describe how Gas Chromatography works and give an application HL - Describe how Mass Spectroscopy works and give an application Infra-red absorption spectrometry (IR) as a ‘fingerprinting’ technique involving absorption of infra-red radiation and give application Ultraviolet absorption spectrometry as a quantitative technique involving the absorption of ultraviolet light and give application Experiment: Separation of a mixture of indicators using paper chromatography or thin-layer chromatography or column chromatography. St.Dominic’s College Chemistry notes Page 6