to - Genentech® Transplant Access Services
advertisement

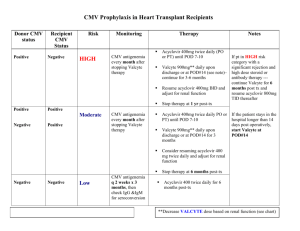
[Date] ATTN: [Medical Review/Appeals] [payer name] [Payer Address] Patient: [Patient’s first and last name] Subscriber ID #: [Insert Subscriber ID #] Subscriber Group #: [Insert Subscriber Group #] RE: Denial of Valcyte (valganciclovir HCl) Dear Appeal Reviewer, Introductory paragraph I am writing this letter of appeal on behalf of my patient, [patient name], who was denied coverage for Valcyte on [date of denial] for [his/her] prophylaxis of CMV disease. The denial reason stated that [give denial reason]. Please see the enclosed documentation that supports the use of this FDA-approved medication. Patient’s Clinical History [Patient’s name] is a [age]-year-old [male/female] who received a [for adults: kidney/heart/kidneypancreas; for pediatrics ages 4 months to 16 years: kidney/heart] transplant on [date]. [Provide rationale for prophylaxis. For example, this may include donor and recipient serostatus, brief description of the patient’s immunosuppressive regimen, and other factors that impact your treatment selection. Include supporting medical records (see Enclosures section below).] Rationale and Treatment Information Valcyte (valganciclovir HCl) is an L-valyl ester (prodrug) of ganciclovir that exists as a mixture of two diastereomers. After oral administration, both diastereomers are rapidly converted to ganciclovir by intestinal and hepatic esterases. [For adults: Valcyte tablets are indicated for the prevention of CMV disease in kidney, heart, or kidney-pancreas transplant patients at high risk (Donor CMV seropositive/Recipient CMV seronegative [D+/R-]). For pediatrics ages 4 months to 16 years: Valcyte for oral solution and tablets are indicated for the prevention of CMV disease in kidney or heart transplant patients (4 months to 16 years of age) at high risk (Donor CMV seropositive/Recipient CMV seronegative [D+/R-]).] It is recommended that prophylaxis start within 10 days of transplantation and continue until [adult heart or kidney-pancreas, or pediatric heart or kidney transplant: 100 days; adult kidney transplant: 200 days] post-transplantation. Conclusion In conclusion, please reconsider the denial of Valcyte for my patient, [patient’s name], for the prophylaxis of CMV disease associated with [his/her kidney/kidney-pancreas/heart transplant]. [Summarize the major points in your letter and restate what you want from the insurance company]. I would appreciate prompt review of this appeal and approval of this FDA-approved therapy. I can be reached at [phone number] for additional information and discussion. Thank you. Sincerely, [Physician Name] Enclosures [Suggested]: Valcyte FDA approval letter & package insert Patient clinical/surgical notes & relevant laboratory reports (including patient/donor serostatus) Denial letter 10537800