Antimicrobial Prophylaxis
advertisement

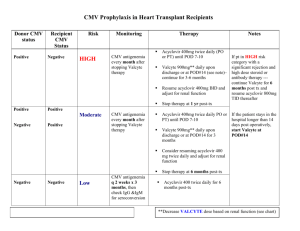
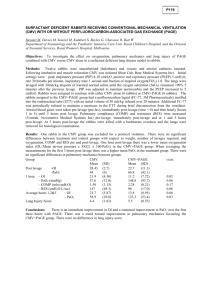
Beth Israel Deaconess Medical Center Transplant Manual Title: Antimicrobial Prophylaxis - Liver Transplantation Purpose: To provide consistent antimicrobial care to liver transplant patients Policy Statement: Liver transplant recipients are at increased risk of bacterial, viral and fungal infections as a consequence of their illness, surgery and immunosuppression. This antimicrobial strategy is used to reduce the risk of infection and decreased morbidity and mortality. 1. 2. Anti-bacterial prophylaxis a. Peri-operative antibiotics (2-5 days) Standard practice Unasyn* 3 g IV q 6 hours Penicillin-allergic Levofloxacin* 500 mg IV q 24 hours Flagyl 500 mg IV q 8 hours Vancomycin* 1 g IV q 24 hours MRSA colonized/hospitalized by >72 hours/admitted from rehab etc. Unasyn* 3 g IV q 6 hours Vancomycin* 1 g IV q 12 hours b. Life-long antibiotics Standard practice Bactrim SS po once daily Sulfa-allergic Nebulized pentamidine 300 mg monthly Anti-viral prophylaxis a. Peri-operative antiviral therapy All patients will get CMV prophylaxis with Valcyte* 450 mg po BID. Donor positive to recipient negative (D+/R- [CMV High Risk]) patients are treated for 6 months and all others are treated for 3 months.** b. All patients not receiving CMV prophylaxis or those finished prophylaxis should have routine surveillance with CMV PCR every 14 days, and CMV IgG for CMV HR patients at month 7,9, and 12 following transplant. c. CMV PCR should be performed every 14 days per month after prophylaxis has been completed. * these drugs require dose adjustment depending on renal function 1 **CMV HR: Valcyte 900 mg po daily in 2 divided doses renally adjusted per standard recommendations. D+/R- (CMV HR): 180 day (6 month) duration standard from time of transplant. IF acute rejection and IS is boosted or patient receives new organ or receives antibody therapy (ATG, OKT3, rituximab), then prophylaxis time is “restarted” and extended for an additional 180 days. D+/R+ or D-/R+ (CMV moderate risk) and D-/R- (CMV low risk): 90 days (3 months) duration standard from time of transplant. IF acute rejection and IS is boosted or patient receives new organ or receives antibody therapy (ATG, OKT3, rituximab), then prophylaxis time is “restarted” and extended for an additional 90 days. 3. Anti-fungal prophylaxis a. b. c All patients listed for liver transplantation receive clotrimazole troche 10 mg po 5 times per day prior to transplantation. Patients will receive fluconazole* 400 mg po x 1 dose pre-transplant. All patients will receive fluconazole* 400 mg po daily for 3 months following transplantation. Vice President Sponsor: Approved by: x Liver Selection Committee Requestor Name: Original Date Approved: Next Review Date: Revised: Dianne Anderson, Sr. VP PCS Douglas W. Hanto, MD, PhD and Michael Curry, MD Co-Chairs Michael Curry, MD 2/02 1/08 Eliminated: 2