organizing elements - introductory periodic table activity
advertisement

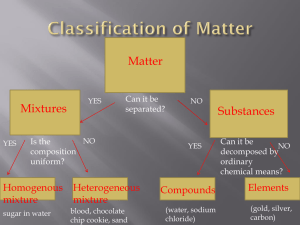
ORGANIZING ELEMENTS Introduction: In this activity you will develop a “min periodic table” based on twenty elements. Your table will be made by grouping elements that share similar physical and chemical properties. To complete the table, you will need to use the table of properties provided (Table #1 & #2) or interpret results of experiments or demonstrations. PART ONE: PHYSICAL PROPERTIES Using tables 1 & 2 circle the elements that have/are (a) (b) (c) (d) Melting points above 2,000 C Boiling points above 700 C Densities above 1.7 g/cm3 in the solid state Gases at 20 C CHEMICAL PROPERTIES Using tables 1 & 2 and the periodic table, circle the elements that are/do (e) Metals (f) Nonmetals (g) Combine with hydrogen to form gaseous or liquid hydrides USE THE RESULTS OF THE EXPERIMENT “CHEMICAL PROERTIES OF SOME METALS” TO COMPLETE ROWS (h) and (i). (h) React with water to produce hydrogen gas (i) React with acids to produce hydrogen gas. USE YOUR OBSERVATIONS OF DEMONSTRATIONS TO COMPLETE ROW (j) (j) Able to form oxides which are acidic when dissolved in water (k) Able to form chlorides with boiling points above 1,000 C and a formula of XCl (l) Able to form chlorides with boiling points above 500 C and a formula of XCl2 (m) At one time were thought to form no compounds and are called noble gases. PART TWO: Using the table of 20 elements you have just circled, place the elements you have determined to have similar properties into groups. Elements should share at least (preferably more) three properties in common to form a group. Try to have the groups of elements be as exclusive as possible (i.e: attempt to have as many groups as possible with the fewest number of elements in each group). For each grouping of elements you develop, list the properties they share in common that caused you to place them together. PERIODIC TABLE WORKSHEET (a) H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca (b) H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca (c) H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca (d) H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca (e) H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca (f) H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca (g) H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca (h) H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca (i) H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca (j) H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca (k) H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca (l) H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca (m) H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca (n) H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca