Liver Liver 22amylouThe Large, Lovable, lackadaisical, Life
advertisement

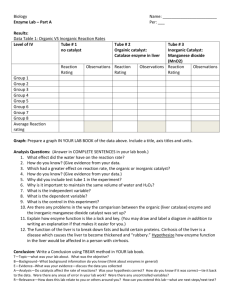
22amylouThe Large, Lovable, lackadaisical, Life-Supporting Organ (Catalyst Lab) Catalysts increase the rate of a chemical reaction without being changed during the reaction. In this experiment, hydrogen peroxide, H2O2, decomposes into oxygen, O2, and water, H2O. An enzyme present in liver cells acts as a catalyst for this reaction. You will investigate the relationship between the amount of catalyst and the rate of the decomposition reaction. Materials: Hydrogen peroxide, 3% Solution Test tube rack with 4 test tubes Mortar and pestle Plastic Spoon Liver cubes, small (3) Graduated Cylinder Scissors Question: How does the amount of surface area of a catalyst affect reaction rate? Hypothesis: Write a statement that answers the question above. Explain your reasoning. Use an If/Then hypothesis. Test the Hypothesis: 1. Using the graduated cylinder, measure 15mL of the hydrogen peroxide solution into each test tube. Make sure that the test tubes are secure in the test tube rack 2. Receive a piece of liver from Mrs. Joshu What are some physical properties of liver? Do NOT taste the liver. 3. Using the scissors, cut the piece of liver into three equal sized pieces- it is okay to estimate a little. 4. Grind one piece of the liver with the mortar and pestle. 5. Using the scissors, cut one piece of the liver into quarters. 6. Leave the last piece of liver as a whole piece. Which “type” of liver do you think: Will react the fastest? Will keep reacting the longest? Will create the fewest bubbles? Is the grossest looking? Produces the most scent? Whole Whole Whole Whole Whole Ground Ground Ground Ground Ground Chunked Chunked Chunked Chunked Chunked 7. At ABOUT the same time, use the spoon to put the liver into three of the test tubes. Leave the fourth test tube with only the hydrogen peroxide in it. What is the purpose of that fourth test tube?? 8. Observe the reaction rate in all four test tubes, and record your observations. (((Be sure to include: Amount of reaction, physical and chemical changes, noticeable temperature changes, etc…))) Whole Liver Test Tube: Observations: Chunked Liver Test Tube: Observations: Ground Liver Test Tube: Observations: Plain Hydrogen Peroxide Test Tube: Observations: Analyze the Results: Does the liver appear to be a catalyst? Explain your answer. Which type of liver (Whole, ground, or chunked) produced a faster reaction? Why? Draw Conclusions: How do your results support or disprove your hypothesis? What are two ways that one could slow down this reaction? What are two ways that one could speed up this reaction?