Liver and Hydrogen Peroxide (Chemical Reactions and Catalysts)
advertisement

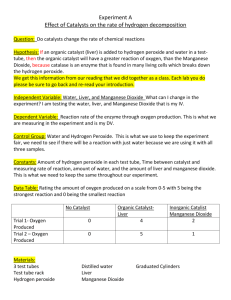
HBio: Enzyme Lab Name:_______________________________Hr:________ Liver and Hydrogen Peroxide (Chemical Reactions and Catalysts) A 1 g piece of fresh liver is placed in a test tube with 1mL of hydrogen peroxide. Bubbles appear in the test tube immediately after the liver is added, which indicates that a gas is produced when the two substances are mixed (see Figure 14.1). The appearance of the gas indicates that a chemical reaction took place inside the test tube. The procedure was then repeated, but this time the gas was captured and tested with a glowing splint. The results of the glowing splint test indicate that the gas was oxygen. (O2). A chemical reaction results from a rearrangement of atoms. When two substances are mixed, however, it is not always clear if the molecules in both substances rearrange, or if the reaction is the result of just one set of molecules being altered in some way. Therefore, in order to be able to explain why oxygen gas is produced when liver is mixed with hydrogen peroxide, you will need to determine what happens to the substance that is in the liver and the molecules of hydrogen peroxide when the two substances are mixed. The guiding question for this investigation is the following: What happens to the substance in the liver that interacts with the hydrogen peroxide when these two substances are mixed? Here are three potential answers to this question: Explanation 1: The substance in the liver that interacts with hydrogen peroxide is altered when the two substances are mixed; the oxygen gas, as a result, comes from the substance in the liver. Explanation 2: The substance in the liver that interacts with hydrogen peroxide is not altered when the two substances are mixed; the oxygen gas, as a result, comes from hydrogen peroxide. Explanation 3: The substance in the liver that interacts with hydrogen peroxide and the hydrogen peroxide are altered when the two substances are mixed; the oxygen gas, as a result, comes from both the hydrogen peroxide and the substance in the liver. Getting Started You can use the following materials to test these three explanations: Test tubes 1 g pieces of fresh liver Hydrogen peroxide Stopwatch Other materials as requested Your task is to determine which explanation provides the best answer to the research question. You can use as many of the supplies available to you to test your ideas. Make sure that you generate the evidence you will need to support your explanation as you work. You can record your method and any observations you will make on the next page. Our Method Our Observations Summary: Which explanation is correct? Explain why it’s correct by describing and analyzing the data you observed.