Real World
advertisement

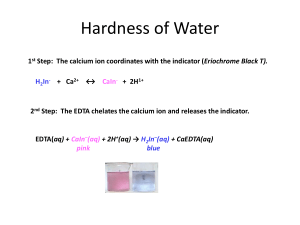
David Millard Naomi Bryner (Partner) Real World: Determining Hardness of Water from Various Sources Introduction: Samples of water from various sources will be tested to determine the concentration of Ca 2+ that each sample contains. A lower limit and upper limit will be found to help determine if the procedure will work. A spike of a known concentration will be added to each sample to prove that the procedure will work. The concentrations will be compared to any water reports that can be found for the samples. Samples will include: Tap water from Brockie Commons, tap water from Brockie Commons filtered through a Brita® water filter, well water (untreated), well water (treated), water from a water fountain (Campbell Hall), Tyler Run Creek, Aquafina®, Dasani®, Fiji®, and Acadia®. Procedure: After collection of the samples, allow all samples to reach room temperatures. Calibrate a pH meter (everyday). Record the pH and temperature of the samples of water that will be tested that day. Create an ammonia buffer with a pH of 10. Prepare an EDTA solution by dissolving 0.7445g of EDTA along with 8mL of ammonia and dilute to 2L Prepare a calcium carbonate solution by dissolving 0.25g of CaCO3 in a 100mL volumetric flask with 0.1M HCl and diluting with 0.1M HCl. o Perform a serial dilution of the CaCO3 solution. Standardize the EDTA solution by titrating with 3mL of the CaCO3 solution, 5mL of ammonia buffer and 10 drops of calmagite indicator. A spike will be added to each sample of water to make sure that the Ca2+ is detectable. o Create a 1000ppm sample of Ca2+ solution and then dilute down to 24ppm. o Add a small sample (1mL) of the 24ppm sample to each sample of water and titrate to make sure that the spike can be recovered. Titrate each sample of water by adding 25mL of water, 3mL of ammonia buffer and 10 drops of calmigite indicator. Data: (Bolded trials were rushed trials and not included in averages) Day 2: Ammonia pH: 9.99, CaCO3 Molarity: 0.01M Standardizing EDTA Trial 1 Trial 2 Trial 3 Trial 4 Average Molarity (M) Standard Deviation Relative Standard Deviation (RSD) (%) EDTA (mL) 30.00 26.69 29.54 29.24 29.49 1.0173*10-3M .2291288 0.77697112 Water fountain water: 7.76 pH, 25°C Trial 1 Trial 2 Trial 3 Trial 4 Trial 5 Average Standard Deviation RSD (%) Ca2+ Concentration Hardness (ppm) EDTA (mL) 21.30 19.61 19.99 19.92 19.80 19.83 0.166333 0.83879475 8.0692*10-4M 32.3396 Brockie Commons (untreated): 7.43 pH, 25°C Trial 1 Trial 2 Trial 3 Average Standard Deviation RSD (%) Ca2+ Concentration Hardness (ppm) EDTA (mL) 19.86 19.58 22.50 20.6467 1.611128 7.8033321 8.4015*10-4M 33.6715 Day 3: Ammonia pH: 10.06, CaCO3 Molarity: 1*10-6M Standardizing EDTA Trial 1 Trial 2 Trial 3 Average Molarity (M) Standard Deviation Relative Standard Deviation (RSD) (%) EDTA (mL) 27.63 28.54 29.13 28.433 1.0551*10-7M .7556675 2.6576816 Acadia bottled water: 6.85 pH, 24°C Spiked Trial 1 Trial 2 Trial 3 Trial 4 Average Standard Deviation RSD (%) 2+ Ca Concentration Hardness (ppm) EDTA (mL) 5.80 5.34 5.28 5.37 5.33 0.0458258 0.8597703 2.2495*10-8M 9.0154*10-4 Without Spike Trial 1 Trial 2 Trial 3 Trial 4 Average Standard Deviation RSD (%) 2+ Ca Concentration Hardness (ppm) EDTA (mL) 5.90 5.00 5.09 5.41 5.167 0.215484 4.1706571 2.1805*10-8M 8.73917*10-4 Without Spike Trial 1 Trial 2 Trial 3 Average Standard Deviation RSD (%) Ca2+ Concentration Hardness (ppm) EDTA (mL) 0.32 0.28 0.59 0.397 0.1686219 42.509714 1.6741*10-9M 6.70942*10-5 Aquafina bottled water: 6.22 pH, 24°C Spiked Trial 1 Trial 2 Trial 3 Average Standard Deviation RSD (%) Ca2+ Concentration Hardness (ppm) EDTA (mL) 0.22 0.52 0.38 0.373 0.1501111 40.208322 1.57656*10-9M 6.31475*10-5 Day 4: Ammonia pH: 10.01, CaCO3 Molarity: 1.76*10-7 Standardizing EDTA Trial 1 Trial 2 Trial 3 Average Molarity (M) Standard Deviation Relative Standard Deviation (RSD) (%) EDTA (mL) 0.39 0.47 0.48 0.4467 1.1821*10-6M 0.0493288 11.043768 Fiji bottled water: 7.30 pH, 24°C Spiked Trial 1 Trial 2 Trial 3 Average Standard Deviation RSD (%) 2+ Ca Concentration Hardness (ppm) EDTA (mL) 28.04 27.21 28.28 27.843 0.5614564 2.0164843 1.3165*10-6M 5.2764*10-2 Without Spike Trial 1 Trial 2 Trial 3 Average Standard Deviation RSD (%) 2+ Ca Concentration Hardness (ppm) EDTA (mL) 26.85 26.42 26.32 26.53 0.2816026 1.0614495 1.2544*10-6M 5.0275*10-2 Without Spike Trial 1 Trial 2 Trial 3 Average Standard Deviation RSD (%) Ca2+ Concentration Hardness (ppm) EDTA (mL) 3.40 3.29 3.59 3.4267 0.1517674 4.4290087 1.6203*10-7M 6.493641*10-3 Dasani bottled water: 6.07 pH, 24°C Spiked Trial 1 Trial 2 Trial 3 Average Standard Deviation RSD (%) Ca2+ Concentration Hardness (ppm) EDTA (mL) 3.34 3.44 3.49 3.423 0.0763763 2.2310495 1.6187*10-7M 6.487324*10-3 Tyler Run Creek: 7.48 pH, 24°C Trial 1 Trial 2 Trial 3 Trial 4 Average Standard Deviation RSD (%) Ca2+ Concentration Hardness (ppm) EDTA (mL) 36.39 37.45 37.50 37.14 37.363 0.1950214 0.5219592 1.7667*10-6M 7.080469*10-2 Brockie Commons (treated): 4.43 pH, 24°C Spiked Trial 1 Trial 2 Trial 3 EDTA (mL) 8.52 8.47 8.51 Average Standard Deviation RSD (%) Ca2+ Concentration Hardness (ppm) 8.50 0.0264575 0.3112649 4.0191*10-7M 1.6107767*10-2 Day 5: Ammonia pH: 10.05, CaCO3 Molarity: 0.0496 Standardizing EDTA Trial 1 Trial 2 Trial 3 Average Molarity (M) Standard Deviation Relative Standard Deviation (RSD) (%) EDTA (mL) 109.40 108.59 110.44 109.4767 1.359*10-3M 0.9273798 0.8471027 Without Spike Trial 1 Trial 2 Trial 3 Trial 4 Average Standard Deviation RSD (%) Ca2+ Concentration Hardness (ppm) EDTA (mL) 9.40 8.09 8.17 8.19 8.15 0.052915 0.6492641 3.8536*10-7M 1.5444506*10-2 Well water (treated): 6.64 pH, 24°C Spiked Trial 1 Trial 2 Trial 3 Average Standard Deviation RSD (%) 2+ Ca Concentration Hardness (ppm) EDTA (mL) 27.74 24.99 24.84 25.857 1.632738 6.3145726 1.40577*10-3M 56.3404 Without Spike Trial 1 Trial 2 Trial 3 Average Standard Deviation RSD (%) 2+ Ca Concentration Hardness (ppm) EDTA (mL) 24.61 24.47 23.99 24.357 0.3251666 1.335021 1.32422*10-3M 53.0720 Well water (untreated): 6.87 pH, 24°C Trial 1 Trial 2 Trial 3 Average Standard Deviation RSD (%) 2+ Ca Concentration Hardness (ppm) EDTA (mL) 27.69 27.42 27.39 27.50 0.1652271 0.6008259 1.49511*10-3M 59.9211 Calculations: Molarity of EDTA M CaCO3 ∗ L CaCO3 = M EDTA L EDTA 0.01M ∗ 0.003L = 0.0010173M 0.02949L Standard Deviation √ ∑(x − x̅)2 n (29.69 − 29.49)2 + (29.59 − 29.49)2 + (29.24 − 29.49)2 √ = 0.2291288 3 Relative Standard Deviation Standard Deviation ∗ 100 x̅ 0.2291288 ∗ 100 = 0.77697112% 24.49 Ca2+ Concentration L EDTA ∗ M EDTA ∗ 0.024350L ∗ 1.359 ∗ 10−3 ∗ mol Ca2+ 1 ∗ = M Ca2+ mol EDTA L Ca2+ 1mol Ca2+ 1 ∗ = 0.00132422M Ca2+ 1mol EDTA 0.025L Ca2+ Hardness of Water M Ca2+ ∗ 0.00132422M ∗ g Ca2+ mg ∗ = ppm Ca2+ 2+ mol Ca g 40.078g Ca2+ 1000mg ∗ = 53.07197511ppm Ca2+ 1mol Ca2+ 1g Conclusion: The main goal of this real world experiment was to determine the Ca2+ concentration and hardness of water in the different samples. We were able to complete this task even with the problems that we had faced with this experiment. The main problem was the lack of information with regards to the water. There was little, to no information when it came to the calcium concentration and hardness of water with some of the samples. Others, like water from the York Water Company and Fiji water, had their information readily available on their website and on the bottle itself.