Experiment 17 Lecture
advertisement

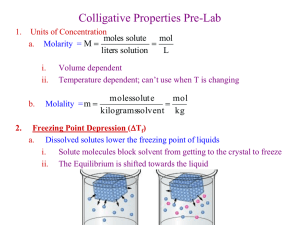
Experiment 17 Lecture Note: I post my lecture notes for these reasons: in case I forgot to mention some crucial points during my actual lecture, in case students did not take notes, and to give students ideas if they are stumped on some part of their lab reports. Please use these notes for ideas, but use your own words when you are writing your discussion section. Do not take a large part of this document, simply change a few words, and use it as your discussion! Colligative Properties Definition/Examples A colligative property is a property of a solution that is caused by the presence of solute molecules and is intensified by an increase in dissolved solute particles. Examples of colligative properties—vapor pressure lowering, boiling point elevation, freezing point depression, osmotic pressure. Example: Vapor pressure lowering (refer to board note photo) o Here is a beaker of pure solvent and a beaker of solution. The circles represent solvent molecules and the X’s represent solute molecules. o Vapor pressure of the solvent comes from the presence of solvent molecules in the gas phase above the liquid surface. In order to vaporize, the molecule must be on the surface. o In a solution, because there are solute molecules, there are less solvent molecules on the surface that can vaporize, and the solute molecules are in the way of solvent molecules trying to reach the surface. o Also, if the solution is not ideal (ideal solutions=interactions between like and unlike molecules are the same), there can be interactions between the solute and solvent that further prevent vaporization. o Because nonvolatile solutes stay in solution instead of volatilizing, they cause greater vapor pressure lowering than volatile solutes. Boiling point elevation can also be understood by this example, and by thinking of the definition of a boiling point. o Boiling point = when the vapor pressure of a liquid is equal to the atmospheric pressure. o Since the vapor pressure for a solution at a given temperature is lower than that for a pure solvent, then in order to achieve a vapor pressure that is equal to atmospheric pressure, the temperature must be higher. Thus, there is an elevated boiling point for the solution vs. the pure solvent. Comparing Phase Diagrams of Solvent and Solution It’s good to think of the effect of colligative properties by looking at the phase diagram of the pure solvent vs. that of the solution. (Refer to board note photo.) o A phase diagram shows the boundary lines between different phases. All points in a phase diagram are points at which multiple phases are present in equilibrium. The Y axis is pressure and the X axis is temperature. o Let’s look at the phase diagram for pure water. We know at least 2 points on this diagram. At 1 atm pressure, the temperature at which ice melts is 0 degrees Celsius. The temperature at which liquid water boils is 100 degrees Celsius. So we have those melting and boiling points for 1 pressure. If we did a bunch of experiments and obtained the melting/freezing and boiling/condensation points at all pressures (as well as the sublimation/deposition points), we would have the phase diagram. o The difference between the liquid/gas boundary of the pure solvent vs. that of the solution is that for the solution, at every temperature, the vapor pressure is lower, so the boundary curve goes down. Also, the melting points are lower, so the solid/liquid boundary is lowered as well. o Now it is easy to see how the presence of solute elevates the boiling points and lowers the freezing points. Freezing Point Depression Equation We will be studying freezing point depression. Here is the freezing point depression equation: ΔTf = -iKfm We want to determine the freezing point depression constant for benzophenone, the solvent we are using. We will measure the freezing points for pure benzophenone and two solutions. o Tf = freezing point of pure solvent o Tf’and T f” = freezing points of the solutions o ΔTf = Tf’ – Tf o m = molality (moles solute/kg solvent) o i = number of dissolved particles per solute molecule or i = moles of dissolved particles per mole solute For nonelectrolyte solutions, i=1. For cyclohexanone, i=1. For electrolyte solutions, you have to determine how many particles will be in solution upon dissolution. For example, if you make an aqueous CaCl2 solution, you will get 3 ions. Cooling Curve Procedure We will measure the freezing points by melting our solvent, then cooling it while measuring the temperature and watching for the formation of solid. o Benzophenone is solid at room temp.; this is why it must be melted before performing the cooling curve procedure. o You will put your benzophenone and solutions in a large test tube. You heat the test tube in a bath of water on a hotplate, using a stir bar as well. o In the test tube, you will put the probe of a digital thermometer and a wire stirrer. o You heat the water until everything in the test tube is liquid and clear. o Then, remove the water bath. As soon as you do this, take the temperature every ten seconds, continuously stir, and watch the test tube for the formation of solid. Note the time at which you see solid form. Then continue to take temperature readings as long as the lab manual says. o You will have a table of data, which you will turn into a graph—a cooling curve. (Refer to the board note photos.) o You will see the temperature of the liquid benzophenone decrease. Then, starting at the time when the solid forms, you will see the temperature plateau. This is because this is when the liquid is converting to solid. The temperature cannot decrease further until all the liquid turns to solid. Then, the solid decreases in temperature. o You can easily trace the freezing temperature by drawing a line back to the Y axis (example below). This is the freezing temperature of benzophenone. You can compare your value to the known value, 47.8 ºC. o You must make sure to keep stirring as you cool, because otherwise you may get supercooling, which is when the temperature of the liquid drops down below the freezing temperature without solidifying. If you do get a supercooling dip, DO NOT trace back from the bottom of the dip—get the freezing point from the level of the solid-liquid plateau. Cooling Curve for Solutions and Extrapolation of F.P. You will then obtain freezing points for two solutions. You will use the same procedure. Note that in order to get the first solution, you simply add 1 mL of solute (cyclohexanone) to the benzophenone from Part 1. And to get the second solution, you just add more benzophenone to the first solution. What will the cooling curve for the first solution look like? You should expect it to be lower than the cooling curve of the pure solvent. Also, it will not have a well-defined phase plateau. The reason for this is because the concentration of the solution will continuously increase (because the benzophenone keeps freezing out of the solution) and thus the temperature of the solution can keep dropping instead of plateauing. Thus, you must extrapolate correctly to get the correct freezing point. o Draw a vertical line at the time where you first saw solid forming. o Then draw a line through the slope of what you think is the solid-liquid transition. Find the intersection of these lines. From there, draw a straight line to the Y axis. This is the freezing point of the solution. What will the cooling curve for the 2nd solution look like? Since we’ve added more solvent, the curve should be in between. So, the freezing point of the pure solvent is highest, followed by solution B, followed by solution A. Solution A has the greatest concentration of solute and so it has the greatest freezing point depression. Once you have determined your freezing points, you can calculate a Kf value from each solution. Since the solvent for both solutions is benzophenone, the K f value you get for both should be about the same. Compare with the known value of K f, 8.7 ºC kg mol-1. Possible errors? 2 groups in each of my classes were not able to melt their benzophenone, no matter how hot or how long they heated it. I believe that they used a bottle of benzophenone that was contaminated with some substance that looked similar to benzophenone but had a much higher melting point. Variations in melting points for the solutions could be caused by not adding enough/adding too much cyclohexanone—the concentration of solute dictates the amount of freezing point depression. Proper use of thermometers in this experiment was important—if you allow a thermometer to touch the sides of the test tube, then it is measuring the test tube’s temperature, not that of the solution. If you do not stir enough while cooling, you may get supercooling, which, especially for the solutions, can make it hard to find an accurate melting point.