Colligative Properties of Solutions
advertisement

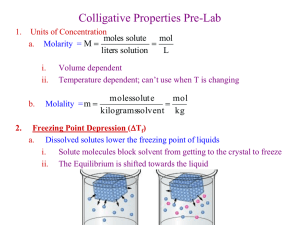
Colligative Properties of Solutions Reaction Rates… How can I make a reaction happen faster? What is a colligative property? • A property that depends on: 1. the concentration of solute particles in solution 2. the solvent used A colligative property does not depend on: 1. the identity of the solute Dilute Versus Concentrated Solutions • Dilute--> a low amount of solute is dissolved in a solvent • Concentrated--> a high amount of solute is dissolved in a solvent • These terms will not tell us whether a solution is saturated, unsaturated, or supersaturated • The concentration of a solution can be found using the Molarity (M) and Molality (m) equations Freezing Point Depression • When a solute is dissolved in a solvent, the freezing point of the solvent will depress (become lower) than the normal freezing point of the pure solute What does the FP depression depend on? • The amount the FP will be lowered depends on 3 factors: 1. The concentration of the solution Higher molality (m) = higher depression 2. Whether or not the solution is an electrolyte Electrolytes = higher depression 3. The identity of the solvent used H2O vs. C2H5OH will exhibit different depressions What is an electrolyte? • A substance that MUST contain ions and conducts electricity (metal & nonmetal) • Electrolytes break apart into + and charged particles which would have a greater effect on the FP depression • Which of the following are examples of electrolytes? NaCl, C12H22O11, CaCl2, C2H5OH How many ions do each of the electrolytes have? How do you find the FP depression of the solvent? ∆Tf = kf n m ∆Tf = FP depression (how many °C lower than the FP of the pure solvent) Kf = FP constant which is dependant on the solvent used (pg. 438 in text) n = number of ions (if applicable) m = molality of the solution Sample Problem • How much will the freezing point be lowered if enough sucrose (C12H22O11) is dissolved in H2O to make a .50 m solution? Kf for H2O = 1.86ºC/m • What if the solute was barium bromide? Calculate the FP depression. • What is the freezing point of a solution containing 16.5 g of sodium chloride in 300. g of acetic acid? Practical Application of FP Depression • Why does putting salt on the roads when it is icy prevent the roads from being slick? The addition of salt as a solute will lower the FP of H2O and help to prevent ice from forming Boiling Point Elevation • When a solute is dissolved in a solvent, the boiling point of the solvent will elevate (become higher) than the normal boiling point of the pure solute • BP elevation depends on the same factors that FP depression does How do you find the BP elevation of the solvent? ∆Tb = kb n m ∆Tb = BP elevation (how many °C higher than the BP of the pure solvent) Kb = BP constant which is dependant on the solvent used (pg. 438 in text) n = number of ions (if applicable) m = molality of the solution Sample Problem • A nonelectrolyte solute is dissolved in 50 kg of benzene (C6H6). The new BP of this solution is 80.61ºC. What is the amount in moles of solute if pure benzene boils at 80.10ºC? • What is the expected boiling point of for a solution that contains 136 g of potassium nitrate in 1.00 kg Practical Application of BP Elevation • Why do cooks add salt to their water when boiling water? • The addition of salt as a solute will force the H2O to boil at a higher temperature which will in turn, cook the food placed in the H2O faster Homework Problems • • • • Pg. 440 #1-4 Pg. 441 #1-4 Pg. 445 #1-3 All of the listed problems above have answers provided • Pg. 449 #19c, 20a, 21a, 21b, 22, 25c, 26a, 30, 31 What Factors Affect the Rate of a Reaction? • 1. Increase in surface area Ex. Increasing the surface area of a substance allows for more particle contact and collisions, therefore increasing the rate of a reaction Granulated sugar should dissolve more quickly in a solvent because of its greater surface area • 2. Increase in temperature Ex. Increasing the temperature of a reaction allows for more frequent particle contact and collisions due to an increase in the kinetic energy of the system, therefore increasing the rate of a reaction • 3. Increase in concentration of reactants Ex. Increasing the concentration of reactants allows for more particle collisions because there are more particles present, therefore increasing the rate of a reaction • 4. Addition of a catalyst Ex. The addition of a catalyst will force the particles to come in contact at a quicker rate, therefore increasing the rate of the reaction • 5. Agitation/Stirring The Collision Theory • In order for a reaction to occur, 2 particles must have an effective collision. A successful collision needs: • 1. Proper orientation (particles must hit in the right spots) • 2. Suitable kinetic energy (particles must hit with the right amount of speed --> too much or too little may result in an ineffective collision) Reaction Rate Diagrams Activation Energy (Ea) = the energy needed to get a reaction started This diagram is representing an exothermic reaction because energy is being released and the products contain less energy than the reactants Lowers Ea Inhibitors increase Ea by slowing down a reaction