Water Hardness Determination Lab Report: EDTA Titration
advertisement
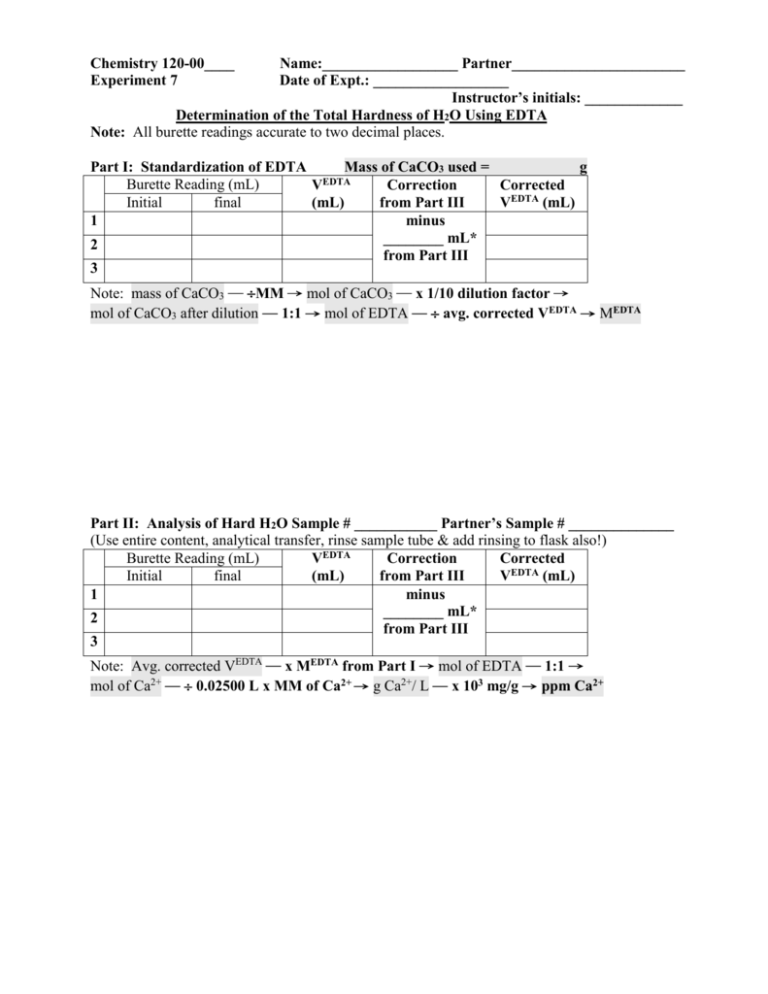
Chemistry 120-00____ Experiment 7 Name:__________________ Partner_______________________ Date of Expt.: __________________ Instructor’s initials: _____________ Determination of the Total Hardness of H2O Using EDTA Note: All burette readings accurate to two decimal places. Part I: Standardization of EDTA Mass of CaCO3 used = g Burette Reading (mL) VEDTA Correction Corrected (mL) from Part III VEDTA (mL) Initial final 1 minus ________ mL* 2 from Part III 3 Note: mass of CaCO3 ─ MM → mol of CaCO3 ─ x 1/10 dilution factor → mol of CaCO3 after dilution ─ 1:1 → mol of EDTA ─ avg. corrected VEDTA → MEDTA Part II: Analysis of Hard H2O Sample # ___________ Partner’s Sample # ______________ (Use entire content, analytical transfer, rinse sample tube & add rinsing to flask also!) Burette Reading (mL) VEDTA Correction Corrected (mL) from Part III VEDTA (mL) Initial final 1 minus ________ mL* 2 from Part III 3 Note: Avg. corrected VEDTA ─ x MEDTA from Part I → mol of EDTA ─ 1:1 → mol of Ca2+ ─ 0.02500 L x MM of Ca2+ → g Ca2+/ L ─ x 103 mg/g → ppm Ca2+ Part III: Correction for Indicator Blanks Burette Reading (mL) VEDTA (mL) Initial final 1 2 Average VEDTA value for Correction = ________ mL* 3 Lab Write Up: 1. Title: Lab 7 Calibration of and EDTA solution and analysis of a hard water sample 2. Procedure: Explain the preparation of your calcium standard and its use to calibrate your EDTA solution. Explain what an indicator blank is and why you have to titrate and calculate this. 3.a. Calculate the concentration of your calcium carbonate standard show your calculations and report your result. 3.b. Calculate the strength of your EDTA solution and report it. 3.c Report the value of your indicator blank. 3.d. Calculate the concentration of your hard water sample in ppm and report the total mg of Ca+2 in your hard water sample and report as: Sample #666 had ______ ppm Ca+2 and ______ mg of Calcium. 4. For 5 % extra credit, analyse Victoria Tap water and report the total hardness using your titration and indicator blank for the calculations. Report the concentration in ppm. Hint it should be low so it is easy to overshoot this.