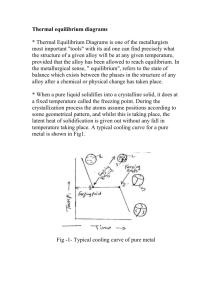
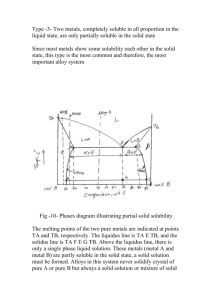
fig diagram
advertisement

Malmö högskola Avd. För Materialvetenskap Ämneskod-linje MT7151 Tentamensdatum 2007-01-09 Skrivtid 14.15-18.15 Tentamen i Tillämpad Materialteknik Examination in Applied Engineering Materials Number of questions: 8 Examiner: Liu-Ying Wei (telephone 57136) Aids: pocket calculator, “ENGLSK-SVENSK ORDLISTA” Summary of questions: I. II. III. IV. V. VI. VII. VIII. Crystalline structure Stress and strain Creep Fe-C system Titanium alloys Ceramics Polymers Precipitation strengthening 5p 9p 10P 12p 13P 6p 4P 7p Total 66P Pass minimum: 30P Mark: 5: >54P; 4: >43P; 3: >30P I. Crystalline structure (5p) 1. Copper (Cu) has a face-centered cubic (fcc) structure, the atomic radius of an Cu atom r = 0.128 nm, calculate the cube edge length “a” of the unit cell. (3p) 2. Sketch a (212) plane and a [0 1 2] direction in a cubic unit cell (see Fig. 1). (2p) z y X Fig. 1 A cubic unit cell 2 II. Stress and strain (9p) Fig. 2 shows the tensile stress-strain behaviour for the brass specimen. The specimen is a cylindrical specimen having an original diameter of 12.8 mm and an original length of 250 mm. Determine the following: 1. The elastic modulus E of the alloy; (2p) 2. The yield strength (Y.S), tensile strength (T.S) and percent elongation of the alloy; (3p) 3. The load applied when the specimen starts to be yielded (1 MPa = 106N/m2); (2p) 4. The change in length of the specimen originally 250 mm long that is subjected to a tensile stress of 345 MPa; (2p) Fig. 2 stress-strain curve of brass 3 III. Creep (10p) 1. A common creep requirement is a 1000h creep life to 2% strain at a (shear) stress of 100 MPa. Calculate the creep rate ( ) for this creep requirement. (Do not forget to give the unit of ) (2p) 2. Predict the time to rupture for a 18-8 Mo stainless steel component that is subjected to a stress of 100MPa at 600ºC (873K). (4p) 3. Consider an 18-8 Mo stainless steel component (Fig. 3) that is exposed to a temperature of 650ºC. What is the maximum allowable stress level for a rupture lifetime tr of one year. (4p) Larson-Miller parameter = T(20+log tr) Fig. 3 Logarithm stress versus the Larson-Miller parameter for an 18-8 Mo stainless steel. 4 VI. Fe-C system (12p) 1. Fig. 4 (a-b) illustrates the microstructures for grey iron and white iron respectively. Give the name of phases pointed by arrows in the pictures. (3p) 2. What is the function of magnesium (Mg) in ductile iron? (2p) Phase 1 Phase 2 Phase 3 b a Fig. 4 Typical microstructures of (a) grey iron; (b) white iron 3. Fe-Fe3C phase diagram is shown in Fig. 5. What phases are to be found in an alloy containing 2 % C (wt %) at (a) 1300 ºC (b) 1000 ºC (c) at 600 ºC.? (3p) 4. A TTT diagram for AISI-SAE 1080 steel (containing 0.8 wt% C) is shown in Fig. 6. Design a process for heat treatment in order to obtain a final microstructure of the 1080 steel containing about 50% fine pearlite (+Fe3C) and 50% martensite. (4p) 5 Fig. 5 Fe-Fe3C phase diagram Fig. 6 TTT diagram for 1080 steel (the dash-line is the 50% completion curve) 6 V. Titanium alloys (13p) 1. What is stabilizer in titanium alloys? Give two examples of the alloying elements for the stabilizer. (2p) 2. A titanium alloy Ti-6Al-4V was subjected to the following heat treatments: (a) held at 1020ºC for 2 hours; (b) quench to 900 ºC and held at 900 ºC for 2h; (c) slowly cooled to room temperature. Using the attached (Ti-6Al)-V phase diagram (Fig. 7) answer the following questions: 1) Give the composition of each phase in the alloy when the alloy has been held at 900 ºC for 2h. (2p) 2) Calculate the amount of each phase in the alloy when the alloy has been held at 900 ºC for 2h (2p) 3) Sketch the microstructure of the alloy when the alloy has been held at 900 ºC for 2h (2p) 4) Sketch the final microstructure of the Ti alloy when it has been slowly cooled down to room temperature; give the chemical composition for each phase in the alloy. (5p) Temperature (ºC) 1000 500 Ms Mf 5 10 Fig. 7 (Ti-6Al)-V phase diagram 7 20 Vanadium wt% VI. Ceramics (6p) 1. What are ceramics? (2p) 2. What is the essential difference between a ceramic and a glass? (2p) 3. What is the basic difference in mechanical properties between ceramic materials and metallic materials? (2p) VII Polymers (4p) 1. What are polymers, give at least four basic characteristics of polymers. (2p) 2. The following is the structure of a monomer for propylene, give the structure of polypropylene (C2H3-CH3)n and the structure of the mer for polypropylene. (2p) 8 VIII. Precipitation strengthening (7p) Cu-containing Al alloys (see phase diagram in Fig. 8) are usually precipitation strengthened. An alloy of 96% Al – 4% Cu has been subjected the following heat treatment: (a) heated up to 550ºC and held at 550ºC for 2 hours; (b) water quenched to room temperature; (c) heated up to 150ºC and held at 150ºC for 20 hours, and then quenched to room temperature. Answer the following questions: 1. describe the microstructure of the alloy after heat treatment (a); (2p) 2. describe the microstructure of the alloy after heat treatment (b); (2p) 3. what happened in stage (c), sketch the final microstructure of the alloy after heat treatment (c). (3p) +L (Al)+ (Al2Cu) Fig. 8 Al-Cu phase diagram 9 10