Homework 2
advertisement

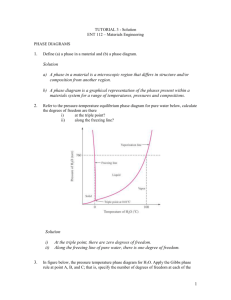
(1) How many triple points are there in the pure iron pressure-temperature equilibrium phase diagram? What phases are in equilibrium at each of the triple points? [10 Points] (2) Consider an alloy containing 70 wt % Ni and 30 wt % Cu (a) At 1350C make a phase analysis assuming equilibrium conditions. In the phase analysis include the following: (i) What phases are present? (ii) What is the chemical composition of each phase? (iii) What amount of each phase is present? (b) Make a similar phase analysis at 1500°C. (c) Sketch the microstructure of the alloy at each of these temperatures by using circular microscopic fields. (3) A Pb-Sn alloy contains 40 wt % and 60 wt % at 50°C. What is the average composition of Pb and Sn in this alloy? (4) Consider the Cu-Zn phase diagram. (a) What is the maximum solid solubility in weight percent of Zn in Cu in the terminal solid solution ? (b) Identify the intermediate phases in the Cu-Zn phase diagram. (c) Identify the three-phase invariant reactions in the Cu-Zn diagram. (i) Determine the composition and temperature coordinates of the invariant reactions. (ii) Write the equations for the invariant reactions. (iii) Name the invariant reactions. (5) Consider the aluminum-nickel (Al-Ni) phase diagram. For this phase diagram: (a) Determine the coordinates of the composition and temperature of the invariant reactions. (b) Write the equations for the three-phase invariant reactions and name them. (c) Label the two-phase regions in the phase diagram.