Honors Chemistry Ka, Kb and Neutralization Rxns. Describe the
advertisement

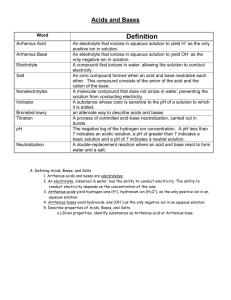
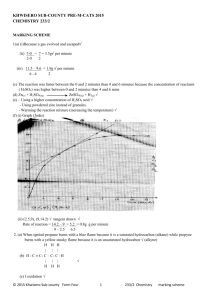
Honors Chemistry Ka, Kb and Neutralization Rxns. 1. Describe the difference between a weak acid and a strong acid. Give an example of each. weak acids partially ionizes in solution, whereas strong acids completely ionize in solution. Weak acid: HC2H3O2 (acetic acid), H2S (hydrosulfic acid). Strong acid: HNO3 (nitric acid), HCl (hydrochloric acid.) 2. Describe the difference between a weak base and a strong base. Give an example of each. weak base partially dissociates in solution and a strong base completely dissociates in solution. Weak base: NH3 ( ammonia), HCO3- (bicarbonate). Strong base: NaOH (sodium hydroxide), KOH (potassium hydroxide.) 3. Define the symbol Ka and Kb. Ka is the acid ionization constant and it is the ratio of the concentrations of the products divided by the [𝐻+][𝐴−] reactants for an acid. If Ka>1 then it is a strong acid and < 1 a weak acid. 𝐾𝑎 = [𝐻𝐴} . Kb is the equivalent relationship for bases. 4. The concentration H+ in a HF acid solution is 1.0 x 10-4 M and the amount of undissociated (not ionized) Hydroflouric Acid, HF, is 2.5 x 10-6 M. What is the dissociation constant for this acid? Is this considered to be a strong or weak acid? + − 𝐻𝐹(𝑔) ⟶ 𝐻(𝑎𝑞) + 𝐹(𝑎𝑞) 𝐾𝑎 = [𝐻+][𝐴−] (1 𝑥 10−4 )(1 𝑥 10−4 ) [𝐻𝐴} 2.5 𝑥10−6 = = .004 therefore weak acid 5. Acetic Acid is the conjugate acid of the acetate anion. It is a weak monoprotic acid that dissociates to an acetate ion and a hydrogen ion in aqueous solution. Calculate Ka for acetic acid if a 1.0 M solution results in an equilibrium [H+] =0.0042 M. + 𝐻𝐶2 𝐻3 𝑂2 ⟶ 𝐻(𝑎𝑞) + 𝐶2 𝐻3 𝑂2 − (𝑎𝑞) 𝐾𝑎 = [𝐻+][𝐴−] (.0042).0042) [𝐻𝐴} 1.0 = = 1.76 𝑥 10−5 therefore weak acid 6. Ammonia is a weak base. If the initial concentration of ammonia is 0.150 M and the equilibrium concentration of OH- is 1.6 x 10-3 M, calculate Kb for ammonia. − 𝑁𝐻3 ⟶ 𝑁𝐻4 + (𝑎𝑞) + 𝑂𝐻(𝑎𝑞) 𝐾𝑏 = [𝑂𝐻−][𝐻𝐵+] (1.6 𝑥 10−3 )(1.6 𝑥 10−3 ) [𝐻𝐵] 0.150 = = 1.71 𝑥 10−5 therefore weak base 7. At 37°C, which is normal body temperature, Kw = 2.4 x 10-14. Calculate [H+] and [OH-] in a neutral solution at this temperature. 𝐾𝑤 = [𝐻 +][𝑂𝐻−] 2.4 x 10-14 = [H+][OH-] 2.4 x 10-14 = x2 = 1.55 x 10-7 M 8. At 50°C, Kw = 5.47 x 10-14. Calculate [H+] and [OH-] in a neutral solution at this temperature. 𝐾𝑤 = [𝐻 +][𝑂𝐻−] 5.47 x 10-14 = [H+][OH-] 5.47 x 10-14 = x2 = 2.34 x 10-7 M 9. A 0.010 M solution of aspirin, a weak monoprotic acid, has a pH of 3.3. What is the Ka of aspirin? 𝑝𝐻 = − log[𝐻 + ] 3.3 = −log[𝐻 + ] 10−3.3 = 5.01 𝑥 10−4 𝑀 𝐾𝑎 = (5.01 𝑥 10−4 )(5.01 𝑥 10−4 ) 0.010 = 2.51 𝑥 10−5 10. A 0.513 M solution of a weak base has a pH of 11.4. What is the Kb of the base? 𝑝𝐻 + 𝑝𝑂𝐻 = 14 𝑝𝑂𝐻 = −log(𝑂𝐻−) pOH=14-11.4 pOH= 2.6 2.6 = −log(𝑂𝐻 − ) 10−2.6 = .0025 𝑀 𝐾𝑏 = (.0025).0025) 0.153 = 4.08 𝑥 10−5 11. Formic acid is a weak monoprotic acid that is partly responsible for the irritation of insect bites. If the initial concentration of formic acid is 0.1 M and the equilibrium concentration of both the H+ and the conjugate base are 4.2 x 10-3, calculate Ka for formic acid. 𝐾𝑎 = (4.2 𝑥 10−3 )(4.2 𝑥 10−3 ) 0.1 = 1.76 𝑥 10−4 12. The acetate ion is a very weak base. If the initial concentration of acetate ion in solution is 0.1 M, and the equilibrium concentration of OH- is 7.5 x 10-6 M, calculate Kb for the acetate ion. 𝐾𝑏 = (7.5 𝑥 10−6 )(7.5 𝑥 10−6 ) 0.1 = 5.63 𝑥 10−10 13. How many moles of LiOH are needed to exactly neutralize 2.0 moles of H2SO4? H2SO4(aq) + 2LiOH(aq) ⟶ 2H2O(l) + Li2SO4(aq) 2.0 𝑚𝑜𝑙𝑒𝑠 𝐻2 𝑆𝑂4 × 2𝑚𝑜𝑙𝑒𝑠 𝐿𝑖𝑂𝐻 = 4.0 𝑚𝑜𝑙𝑒𝑠 𝐿𝑖𝑂𝐻 1 𝑚𝑜𝑙𝑒𝑠 𝐻2 𝑆𝑂4 14. How many moles of H2SO4 are needed to neutralize 5.0 moles of NaOH? H2SO4(aq) + 2NaOH(aq) ⟶ 2H2O(l) + Na2SO4(aq) 5.0 𝑚𝑜𝑙𝑒𝑠 𝑁𝑎𝑂𝐻 × 1 𝑚𝑜𝑙𝑒𝑠 𝐻2 𝑆𝑂4 = 2.5 𝑚𝑜𝑙𝑒𝑠 𝐻2 𝑆𝑂4 2 𝑚𝑜𝑙𝑒𝑠 𝑁𝑎𝑂𝐻 15. How many moles of HCL are needed to neutralize 0.10 L of 2.0 M NaOH? HCl(aq) + NaOH(aq) ⟶ H2O(l) + NaCl(aq) 2.0 𝑚𝑜𝑙𝑒𝑠 𝑁𝑎𝑂𝐻 1 𝑚𝑜𝑙 𝐻𝐶𝑙 0.10𝐿 × × = 0.2 𝑚𝑜𝑙 𝐻𝐶𝑙 1𝐿 1 𝑚𝑜𝑙 𝑁𝑎𝑂𝐻 1 16. How many moles of NaOH are needed to neutralize 0.010 L of 0.20 M H2SO4? H2SO4(aq) + 2NaOH(aq) ⟶ 2H2O(l) + Na2SO4(aq) 0.20 𝑚𝑜𝑙𝑒𝑠 𝐻2 𝑆𝑂4 2 𝑚𝑜𝑙 𝑁𝑎𝑂𝐻 0.10𝐿 × × = 0.04 𝑚𝑜𝑙 𝑁𝑎𝑂𝐻 1𝐿 1 𝑚𝑜𝑙 𝐻2 𝑆𝑂4 1 17. If it takes 15.0 mL of 0.40 M NaOH to neutralize 5.0 mL of HCl, what is the molar concentration of the HCl solution? HCl(aq) + NaOH(aq) ⟶ H2O(l) + NaCl(aq) 0.40 𝑚𝑜𝑙𝑒𝑠 𝑁𝑎𝑂𝐻 1 𝑚𝑜𝑙 𝐻𝐶𝑙 0.015𝐿 1 × × × = 1.2 𝑀 𝐻𝐶𝑙 1𝐿 1 𝑚𝑜𝑙 𝑁𝑎𝑂𝐻 1 0.005𝐿 18. If it takes 10.0 mL of 2.0 M H2SO4 to neutralize 30.0 mL of KOH, what is the molar concentration of the KOH? H2SO4(aq) + 2KOH(aq) ⟶ 2H2O(l) + K2SO4(aq) 2.0 𝑚𝑜𝑙𝑒𝑠 𝐻2 𝑆𝑂4 2 𝑚𝑜𝑙 𝐾𝑂𝐻 0.010𝐿 1 × × × = 1.33 𝑀 𝐾𝑂𝐻 1𝐿 1 𝑚𝑜𝑙 𝐻2 𝑆𝑂4 1 0.030𝐿 19. How many mL of 2.0 M H2SO4 are required to neutralize 30.0 mL of 1.0 M NaOH? H2SO4(aq) + 2NaOH(aq) ⟶ 2H2O(l) + Na2SO4(aq) 1.0 𝑚𝑜𝑙𝑒 𝑁𝑎𝑂𝐻 1 𝑚𝑜𝑙 𝐻2 𝑆𝑂4 0.03𝐿 1𝐿 × × × = .0075 𝐿 𝑜𝑟 7.5 𝑚𝐿 1𝐿 2 𝑚𝑜𝑙 𝑁𝑎𝑂𝐻 1 2.0 𝑚𝑜𝑙 𝐻2 𝑆𝑂4 20. How many mL of 0.10 M Ca(OH)2 are required to neutralize 25.0 mL of 0.50 M HNO3? 2HNO3(aq) +Ca(OH)2(aq) ⟶ 2H2O(l) + Ca(NO3)2(aq) 0.5 𝑚𝑜𝑙𝑒𝑠 𝐻𝑁𝑂3 1 𝑚𝑜𝑙 𝐶𝑎(𝑂𝐻)2 0.025𝐿 1𝐿 × × × = .0625 𝐿 𝑜𝑟 62.5 𝑚𝐿 1𝐿 2 𝑚𝑜𝑙 𝐻𝑁𝑂3 1 0.1 𝑚𝑜𝑙 𝐶𝑎(𝑂𝐻)2