Study Guide Solutions Acids Bases Answer Key
advertisement
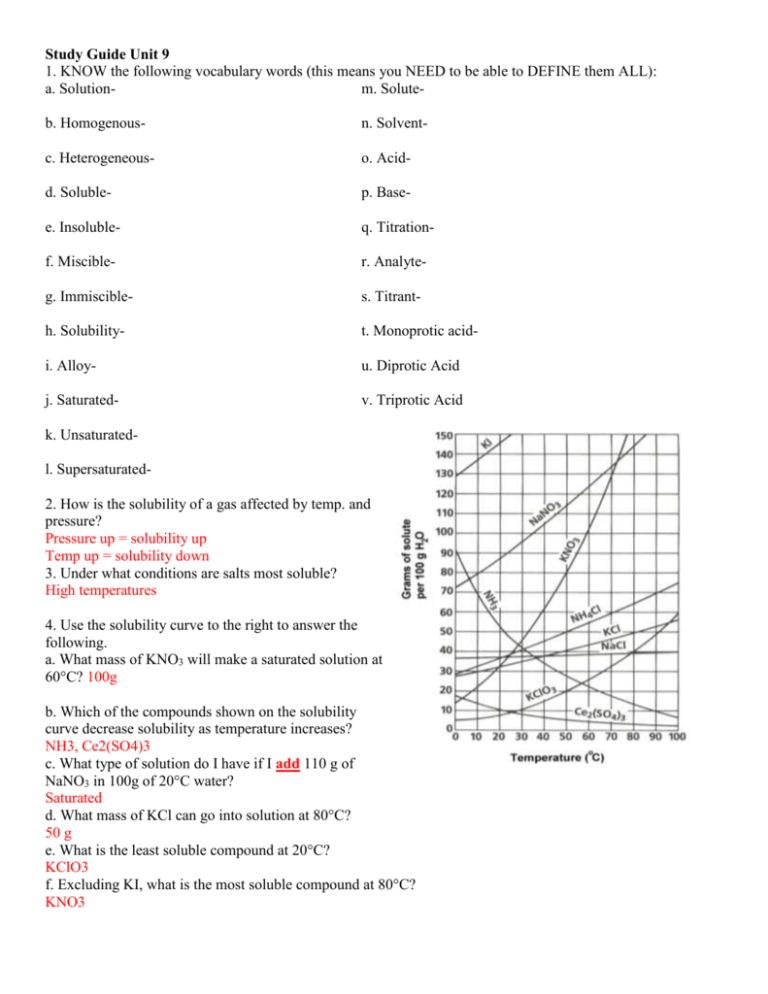
Study Guide Unit 9 1. KNOW the following vocabulary words (this means you NEED to be able to DEFINE them ALL): a. Solutionm. Soluteb. Homogenous- n. Solvent- c. Heterogeneous- o. Acid- d. Soluble- p. Base- e. Insoluble- q. Titration- f. Miscible- r. Analyte- g. Immiscible- s. Titrant- h. Solubility- t. Monoprotic acid- i. Alloy- u. Diprotic Acid j. Saturated- v. Triprotic Acid k. Unsaturatedl. Supersaturated2. How is the solubility of a gas affected by temp. and pressure? Pressure up = solubility up Temp up = solubility down 3. Under what conditions are salts most soluble? High temperatures 4. Use the solubility curve to the right to answer the following. a. What mass of KNO3 will make a saturated solution at 60°C? 100g b. Which of the compounds shown on the solubility curve decrease solubility as temperature increases? NH3, Ce2(SO4)3 c. What type of solution do I have if I add 110 g of NaNO3 in 100g of 20°C water? Saturated d. What mass of KCl can go into solution at 80°C? 50 g e. What is the least soluble compound at 20°C? KClO3 f. Excluding KI, what is the most soluble compound at 80°C? KNO3 Study Guide Unit 9 5. What is the molarity of 0.629 moles of Al2O3 to make 1.500 liters of solution? 0.419 M 6. How many moles of Na2CO3 are in 10.0 mL of a 2.0 M solution? 0.02 moles 7. How many moles of NaCl are contained in 100.0 mL of a 0.20 M solution? 0.02 moles 8. What mass of NaCl would be contained in problem 7? 1.17 g 9. What mass of H2SO4 would be needed to make 750.0 mL of 2.00 M solution? 147 g 10. What volume (in mL) of 18.0 M H2SO4 is needed to contain 2.45 g H2SO4? 1.38 mL 11. What volume (in mL) of 12.0 M HCl is needed to contain 3.00 moles of HCl? 25 mL 12. How many grams of Ca(OH)2 are needed to make 100.0 mL of 0.250 M solution? 1.85 g 8. What type of compound will conduct electricity when dissolved in water? ionic 9. If I dissolve 40 g of NaCl in water how will it affect the heating curve below? Draw the changes. 10. Which of the following will conduct electricity when dissolved in water: LiF, Na2SO4, CH4, CH3OH, SO3, MgI2, SrCl2 11. Why is salt added to the roads during winter? To keep them from freezing Study Guide Unit 9 12. What does the formula of an acid start with? H 13. What does the formula of a base end in? OH 14. What is the pH range of an acid and a base? 0-14 15. What are some properties of acids and bases? See notes 16. Name the following acids: a. HBr Hydrobromic d. H2CO3 Carbonic Acid b. HCl Hydrochloric e. HI Hydroiodic acid c. HNO3 Nitric Acid f. H2SO4 Sulfuric Acid 17. Write the formula for the following acids: a. Sulfuric Acid H2SO4 d. Carbonic acid . HCl b. Hydrochloric acid HCl e. Hydroiodic acid HI c. Acetic acid HC2H3O2, HC2H3OO 18. What is the pH of a 0.045 M solution of HCl? 1.35 f. Nitric acid HNO3 19. What is the pH of a 0.089 M solution of LiOH? 12.95 20. What is the concentration of [H+] in a solution of HClO3 with a pH of 5.5? 3.16E-6 21. What is the pOH of a 0.23 M solution of HI? 13.36 22. What is the concentration of [OH-] in a solution of Mg(OH)2 with a pH of 7.1? 7.94E-8 23. What is the pOH of a 0.65 M solution of LiOH? 0.19 24. What is the concentration of [OH-] in a solution of Ca(OH)2 with a pH of 8.4? 2.51E-6 25. What is the pOH of a 0.013 M solution of NaOH? 1.89 26. What is the pH of a 0.067 M solution of KOH? 12.83 Study Guide Unit 9 27. What is the pH of a 0.023 M solution of HNO3? 1.64 28. What is the concentration of [H+] in a solution of HBr with a pH of 3.5? 3.16E-4 29. Determine the hydrogen ion concentration of 0.780 M solution of RbOH. 0.78 30. Predict the products of the following reactions: a. ____HI + _____ NaOH-> H2O + NaI b. __3__Ca(OH)2 + ___2__ H3PO4 -> 6 H2O + Ca3(PO4)2 c. _____H2CO3 + _____Sr(OH)2 -> 2 H2O + SrCO3 d. _____Zn(OH)2 + ___2__HNO3-> 2 H2O + Zn(NO3)2 30. 160 mL of 2.2 M NaOH are required to titrate 140 ml of HF. What is the M of the HF? 2.51 31. What volume of 0.40 M NaOH would be required to titrate 17.0 mL of 0.15 M HCl? 6.38 32. 140 mL of 3.1M H2SO4 are required to titrate 50 mL of NaOH to the equivalence point. What is the M of the NaOH? 8.68 33. 85 mL of 2.5M H2SO4 are required to titrate 20 mL of NaOH to the equivalence point. What is the M of the NaOH? 10.63











