Day 1: Properties of Arrhenius Acids and Bases
advertisement
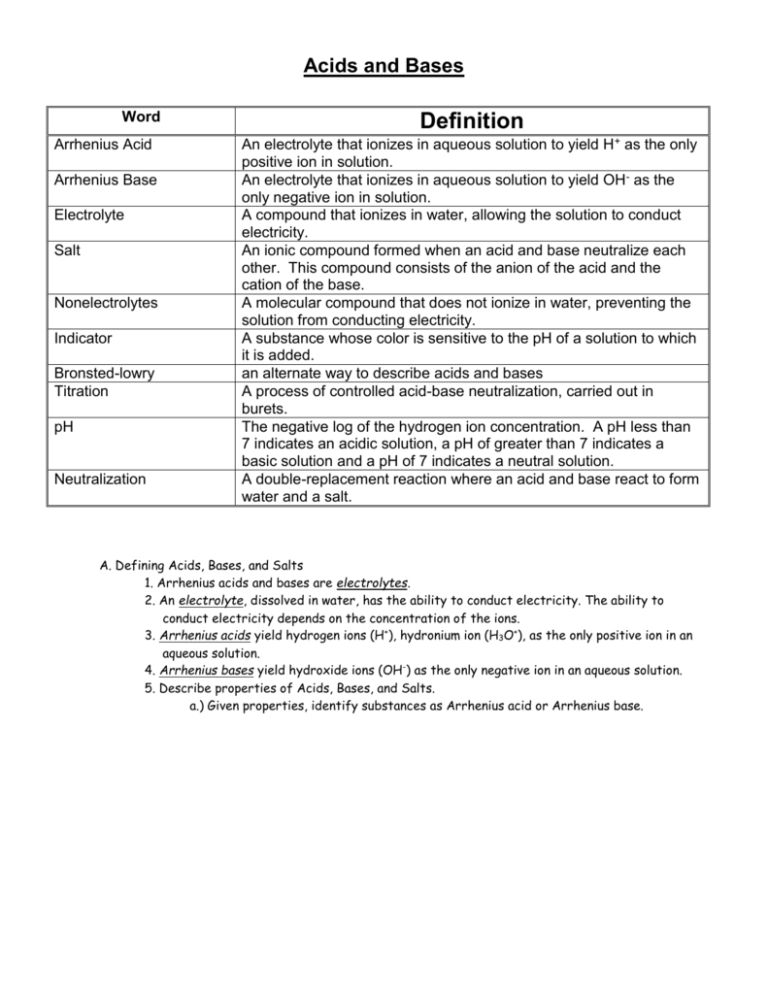
Acids and Bases Word Arrhenius Acid Arrhenius Base Electrolyte Salt Nonelectrolytes Indicator Bronsted-lowry Titration pH Neutralization Definition An electrolyte that ionizes in aqueous solution to yield H+ as the only positive ion in solution. An electrolyte that ionizes in aqueous solution to yield OH- as the only negative ion in solution. A compound that ionizes in water, allowing the solution to conduct electricity. An ionic compound formed when an acid and base neutralize each other. This compound consists of the anion of the acid and the cation of the base. A molecular compound that does not ionize in water, preventing the solution from conducting electricity. A substance whose color is sensitive to the pH of a solution to which it is added. an alternate way to describe acids and bases A process of controlled acid-base neutralization, carried out in burets. The negative log of the hydrogen ion concentration. A pH less than 7 indicates an acidic solution, a pH of greater than 7 indicates a basic solution and a pH of 7 indicates a neutral solution. A double-replacement reaction where an acid and base react to form water and a salt. A. Defining Acids, Bases, and Salts 1. Arrhenius acids and bases are electrolytes. 2. An electrolyte, dissolved in water, has the ability to conduct electricity. The ability to conduct electricity depends on the concentration of the ions. 3. Arrhenius acids yield hydrogen ions (H+), hydronium ion (H3O+), as the only positive ion in an aqueous solution. 4. Arrhenius bases yield hydroxide ions (OH-) as the only negative ion in an aqueous solution. 5. Describe properties of Acids, Bases, and Salts. a.) Given properties, identify substances as Arrhenius acid or Arrhenius base. Acid Properties Base Properties pH: less than 7 greater than 7 Taste: Sour Bitter Reacts w/Metal Yes (metals must be above **H2 on Ref Table J) Effect on Litmus Paper Turns Red Effect on Phenolpthalein Colorless No Turns Blue Pink 6. Bronted-Lowry theory states that an acid is a proton donor and a base is a proton acceptor. 7. The acidity or alkalinity(basicity) of a solution can be measured by its pH value. 8. On the pH scale, each decrease of one unit of pH indicates a tenfold increase in the hydronium ion concentration. a.) Identify solution as acid, base, or neutral based upon the pH. Acids = pH > 7 (More H+/H3O+ ions than OH- ions) Bases = pH < 7(More OH- ions than H+/H3O+ ions) Neutral = pH = 7 (Equal H+/H3O+ ions and OH- ions) 9. The relative level of acidity in a solution can be shown with indicators. Interpret changes in acid-base indicator color. Reference Table M B. Reactions of Acids and Bases 1. In the process of neutralization, an acid and a base react to form a salt and water. a.) Write simple neutralization reactions when given the reactants. Double Replacement Reaction Acid + Base Salt + H2O 2. Titration is a process in which a volume of solution of known concentration is used to determine the concentration of another solution. 3. Calculate the molarity of an acid or base. 4. Solve titration problems. a.) Calculate the concentration, or volume of a solution, using titration data. MAVA=MBVB 1) ACIDS AND BASES HOMEWORK A) Identify each as an acid or base based on their formulas and properties. Property Acid or Base? Property Acid or Base? Turns litmus red Acid Turns phenolphthalein clear Acid Tastes sour Acid Tastes bitter Base Hydrolyzes fats into soap Base Reacts with active metals to form H2 Acid HCl (aq) Acid KOH (aq) Base pH of 12 Base Forms H3O+ in water Acid B) Which of the following metals will react in the presence of an acid? 1) Cu b) Ag c) Zn d) Au C) Name the following acids: 1) HCl (aq) ___Hydrochloric Acid___________________________________ 4) HNO3 (aq) ____Nitric Acid__________________________________________ 3) H2SO4 (aq) ____Sulfuric Acid________________________________________ 4) HBr (aq) ____Hydrobromic Acid___________________________________ 5) HC2H3O2 (aq)___Acetic Acid_________________________________________ D) Write the formulas of the following acids: 1) Hydrofluoric acid ____HF____________________________________ 2) Phosphoric acid ____H3PO4____________________________________ 3) Hydrobromic acid ____HBr____________________________________ E) Name the following bases: 1) KOH (aq)___Potassium Hydroxide____________________ 2) Ca(OH)2 (aq)___Calcium Hydroxide___________________ 3) LiOH (aq)___Lithium Hydroxide_______________________ F) Write the formulas for the following bases: 1) aluminum hydroxide____Al(OH)3_____________________________ 2) barium hydroxide____Ba(OH)2________________________________ 3) sodium hydroxide____NaOH________________________________ G) What is the pH of a solution that turns methyl orange to yellow, phenolphthalein to clear, bromcresol green to blue and thymol blue to yellow? a) 2.7 b) 5.6 c) 9.0 d) 10.2 Explain how you arrived at your answer, using the process of elimination to show how you used Reference Table M to eliminate each choice. If methyl orange is yellow, then the pH is higher than 4.4 and choice A can be eliminated. If phenolphthalein is clear then the pH is lower than 8.2 and choices C and D can be eliminated. If bromcresol green is blue then the pH is higher than 5.4 and choice A can be eliminated. If thymol blue is yellow then the pH is lower than 8.0 and choices C and D can be eliminated. 2) Acid and Base Neutralization Homework A) Write the formula of the salt formed in each reaction: 1) H2SO4 (aq) + Mg(OH)2 (aq) 2 HOH + __MgSO4_______________ 2) H3PO4 (aq) + 3 NaOH (aq) 3 HOH + ___Na3PO4_______________ 3) H2CO3 (aq) + 2 KOH (aq) 2 HOH + ____K2CO3_______________ 4) H2SO4 (aq) + 2 KOH (aq) 2 HOH + ____K2SO4_______________ B) Write the formula of the acid used in each reaction: 1) 3 ___H2SO4_________________ (aq) + 2 Al(OH)3 (aq) 6 HOH (l) + Al2(SO4)3 (aq) 2) 2 ____HC2H3O2________________ (aq) + Ba(OH)2 (aq) 2 HOH (l) + Ba(C2H3O2)2 (aq) 3) ____H2CO3__________________(aq) + 2 NH4OH (aq) 2 HOH + (NH4)2CO3 C) Write the formula of each base used in each reaction: 8) H2SO3 (aq) + ___Mg(OH)2__________ (aq) 2 HOH (l) + MgSO3 (aq) 9) 2 H3PO4 (aq) + 3 __Ca(OH)2__________ (aq) 6 HOH (l) + Ca3(PO4)2 (aq) 10) 2 HCl + ___Ca(OH)2________ CaCl2 (aq) + 2 HOH (l) C) Solve the following titration problems: 1) How many moles of KOH are needed to neutralize 1.5 moles of H2SO4? 3.0 moles KOH 2) How many moles of NaOH are needed to neutralize 3.0 moles of HCl? 3.0 moles NaOH 3) How many moles of H3PO4 are required to exactly neutralize 5.0 moles of KOH? 1.7 moles H3PO4 4) What volume of 0.80 M HCl will exactly neutralize 100. mL of 0.40 M KOH? 50 mL of 0.80 HCl 5) If exactly 5.0 mL of HNO3 will neutralize 15 mL of 2.0 M NaOH, what is the molarity of the HNO 3 solution? 6 M HNO3 6) What volume of 0.25M HCl is needed to neutralize 1.5 L of 4.0 M NaOH? 24 L of 0.25 M HCl 7) What volume of 5.0 M NaOH is needed to neutralize 40. mL of 2.0 M HCl? 16 mL of 5.0 M NaOH 8) What is the molarity of an H2CO3 solution if it takes 50. mL of H2CO3 to exactly neutralize 100. mL of 0.50 M NaOH? 0.50 M H2CO3 9) What is the molarity of a NaOH solution if it takes 100. mL of NaOH to neutralize 50. mL of 0.10 M H 2SO4? 0.1 M NaOH 10) What is the molarity of a 2.0 L sample of HCl that exactly neutralizes 1.0 L of a 2.00 M sample of NaOH? 1.0 M HCl 11) 50. mL of H2SO4 of unknown concentration is titrated with 25 mL of 5.0 M NaOH. What is the molarity of the H2SO4? 1.25 M H2SO4 12) How many mL of 0.50 M HCl are required to neutralize 30. mL of 2.0 M Mg(OH) 2? 240 mL of 0.50 M HCl 13) How many mL of 1.0 M H3PO4 are required to neutralize 50. mL of 3.0 M NaOH? 50 mL of 1.0 M H3PO4 14) 200. mL of KOH are required to neutralize 100. mL of 0.10 M H 2SO4. What is the molarity of the KOH? 0.025 M KOH 15) How many moles of HCl can be neutralized by 0.50 L of 0.10 M NaOH? 0.05 moles of HCl 16) How many moles of H2SO4 can be neutralized by 0.10 L of 0.50 M NaOH? 0.025 moles of H2SO4 3) The Power of Hydronium Homework 1) A pH of 4 is how many times more acidic than a pH of 6? 100 times more acidic 2) A pH of 11 is how many times more basic than a pH of 8? 1000 times more basic 3) Which of the following solution pH values is the most acidic? a) 5 b) 7 c) 9 d) 11 4) A pH of 14 is how many times more basic than a pH of 12? a) 10 times b) 100 times c) 1000 times b) 10000 times 5) Which of the following solutions can have a pH of 5? a) a 10-5 M solution of HCl b) a 10-5 M solution of NaOH c) a 10-5 M solution of NaCl d) a 10-5 M solution of CH4 6) What is the color of methyl orange in a solution whose pH is 9? Yellow Is this an acid or a base? Base 7) What is the color of litmus in a solution whose pH is 10? Blue Is this an acid or a base? Base 8) What is the color of bromcresol green in a solution with a pH of 2? Yellow Is this an acid or a base? Acid 9) What is the color of thymol blue in a solution with a pH of 13? Blue Is this an acid or a base? Base 10) What is the pH of a solution formed when equal volumes of 0.1 M HCl and 0.1 M NaOH are added to a beaker? a) less than 7 b) more than 7 c) exactly 7 4) Alternate Theory of Acids and Bases For each of the following systems, identify the acid and base: 1. A HBr + B CA H2O <====> H3O+ 2. A or B H2O + A or B H2O <====> CA H3O+ + 3. A NH3 + B OH- <====> CB NH2- 4. B H2O + A HPO42- 5. A H3PO4 6. B CH3COO- + A H3O+ 7. A H2PO4- + B CH3COO- <====> 8. A H2O + B S2- 9. B CN- + A HCH3COO 10. B OH- + A NH4+ + CB Br- + CB OH- + CB PO43- <====> B H2O <====> <====> <====> <====> CA H3O+ + CB H2PO4- + + CA HCH3COO <====> CA HS- CA H2O CA HCH3COO + CA H3O+ + CB HPO42- CB OHCA HCN CA H2O CB CH3COO- + + CB NH3 CB H2O








