Dilutions, Solution
Stoichiometry and
Titrations
Copyright © 2008 Pearson Prentice Hall, Inc.
Chapter 1/1
Diluting Concentrated
Solutions
concentrated solution + solvent
dilute solution
initial final
Mi Vi = Mf Vf
Since the number of moles of solute remains constant,
all that changes is the volume of solution by adding
more solvent.
Copyright © 2008 Pearson Prentice Hall, Inc.
Chapter 3/2
Diluting Concentrated
Solutions
Sulfuric acid is normally purchased at a concentration of
18.0 M. How would you prepare 250.0 mL of 0.500 M
aqueous H2SO4?
Mi = 18.0 M
Mf = 0.500 M
Vi = ? mL
Vf = 250.0 mL
Vi =
Mf Vf
Mi
0.500 M
=
18.0 M
250.0 mL
x
= 6.94 mL
Add 6.94 mL 18.0 M sulfuric acid to enough water to
make 250.0 mL of 0.500 M solution. Why?
Copyright © 2008 Pearson Prentice Hall, Inc.
Chapter 3/6
Solution Stoichiometry
aA + bB
Volume of
Solution of A
Moles of
A
Molarity of
A
cC + dD
Moles of
B
Mole Ratio
Between A
and B
(Coefficients)
Volume of
Solution of B
Molar Mass
of B
Copyright © 2008 Pearson Prentice Hall, Inc.
Chapter 3/11
Solution Stoichiometry
What volume of 0.250 M H2SO4 is needed to react with
50.0 mL of 0.100 M NaOH?
H2SO4(aq) + 2NaOH(aq)
Volume of
Solution of H2SO4
Moles of
H2SO4
Molarity of
H2SO4
Na2SO4(aq) + 2H2O(l)
Moles of
NaOH
Mole Ratio
Between H2SO4
and NaOH
Volume of
Solution of NaOH
Molarity of
NaOH
Copyright © 2008 Pearson Prentice Hall, Inc.
Chapter 3/12
Solution Stoichiometry
H2SO4(aq) + 2NaOH(aq)
Na2SO4(aq) + 2H2O(l)
Moles of NaOH available:
50.0 mL NaOH
0.100 mol
x
1L
1L
x
1000 mL
= 0.00500 mol NaOH
Volume of H2SO4 needed:
0.00500 mol NaOH 1 mol H2SO4
1 L solution
1000 mL
x
x
x
2 mol NaOH 0.250 mol H2SO4
1L
10.0 mL solution (0.250 M H2SO4)
Copyright © 2008 Pearson Prentice Hall, Inc.
Chapter 3/13
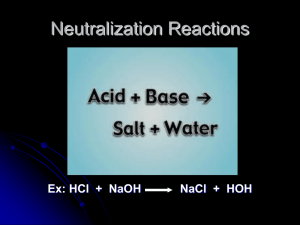
Titration
Titration: A procedure for determining the concentration
of a solution by allowing a carefully measured volume to
react with a solution of another substance (the standard
solution) whose concentration is known.
HCl(aq) + NaOH(aq)
NaCl(aq) + 2H2O(l)
Once the reaction is complete you can calculate the
concentration of the unknown solution.
How can you tell when the reaction is complete?
Copyright © 2008 Pearson Prentice Hall, Inc.
Chapter 3/25
Titration
buret
Erlenmeyer
flask
standard solution
(known concentration)
unknown concentration solution
An indicator is added which changes
color once the reaction is complete
Copyright © 2008 Pearson Prentice Hall, Inc.
Chapter 3/26
Titration
48.6 mL of a 0.100 M NaOH solution is needed to react
with 20.0 mL of an unknown HCl concentration. What is
the concentration of the HCl solution?
HCl(aq) + NaOH(aq)
Volume of
Solution of NaOH
Moles of
NaOH
Molarity of
NaOH
NaCl(aq) + 2H2O(l)
Moles of
HCl
Mole Ratio
Between NaOH
and HCl
Volume of
Solution of HCl
Molarity of
HCl
Copyright © 2008 Pearson Prentice Hall, Inc.
Chapter 3/31
Titration
HCl(aq) + NaOH(aq)
NaCl(aq) + 2H2O(l)
Moles of NaOH available:
48.6 mL NaOH 0.100 mol
1L
x
x
= 0.00486 mol NaOH
1L
1000 mL
Moles of HCl reacted:
0.00486 mol NaOH 1 mol HCl
= 0.00486 mol HCl
x
1 mol NaOH
Concentration of HCl solution:
0.00486 mol HCl
20.0 mL solution
1000 mL
x
1L
= 0.243 M HCl
Copyright © 2008 Pearson Prentice Hall, Inc.
Chapter 3/32
H12 – C3
• 3.19, 3.20, 3.22, 3.87*, 3.89, 3.91*,
3.93*, 3.120*









![pH = - log [H + ]](http://s2.studylib.net/store/data/005622524_1-002df1ea50d2a849b15deb604928664e-300x300.png)