Acid-Base Titration Worksheet: Molarity & Normality Calculations
advertisement

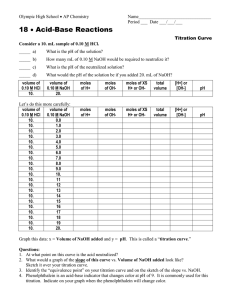
ACID-BASE TITRATION The process of adding measured volumes of acid or base of known concentration to an acid or base of unknown concentration until neutralization occurs is called titration. The known concentration of acid or base is referred to as the standard solution. The point at which the neutralization has occurred is called the end point. The end point is usually reached when a color change is noted. The color change is produced by using an acid base indicator (usually phenolphthalein) that changes color when an acid or base is present. The unknown concentration or volume can be calculated from the known volumes and the known concentration of the standard solution. Remember that it is the hydrogen or hydroxide ions that are responsible for the acidic and basic properties, so if we want to achieve neutrality, we have to have a 1:1 ratio of moles of H + to moles of OH-. What concentration calculation can we do to relate moles and volume? Molarity Take a look at the titration equation on the reference table: NaVa = NbVb, where N = Normality of the acid or base. Normality is defined as the concentration of the H+ or OH- ions, so the equation = Molarity of H+ x Volume of acid = Molarity of OH- x Volume of base Here’s how we use this: SAMPLE PROBLEM How many milliliters of 0.2 M KOH are needed to neutralize 20 mL of 0.1 M HCl? NbVb = NaVa (0.1 M) (20mL) = (0.2 M) (Vb) 2 = .2 x 2 = 10 mL .2 Look at this though: How many moles of H+ are in 1 mole of HCl? This ratio makes HCl a monoprotic acid And how many moles of OH- are in 1 mole of KOH? So this is a monohydroxy base. 1 1 In these cases the normality of the whole substance is equal to the molarity of the ion that we’re interested in. This isn’t always true though. Look at sulfuric acid, H2SO4; how many moles of H+ are there per mole of acid? 2 Can you guess what type of acid sulfuric is considered then? Diprotic What type of base is Mg(OH)2? Dihydroxy If you know that you have a 2M solution of Mg(OH)2, what would have have to do to get the normality? Multiply the molarity by 2 So you have to take this in to account when using the titration formula. Here’s an example: How much 3.0 M H2SO4 is needed to neutralize 50.mL of 1.2 M Al(OH)3? Step 1: Determine the concentrations of H+ and OH-: NA = 3.0 M × 2 = 6.0 M NB = 1.2 M × 3 = 3.6 M Step 2: Substitute values into the equation and solve for the unknown: NA × VA = NB × VB (6.0 M) VA = (3.6 M)(50. mL) VA = 30. mL So we would need to use 30ml of 3M H2SO4 to neutralize 50.mL of 1.2 M Al(OH)3. Here is a worksheet for you to get some practice with this. You are going to use this knowledge to determine whether or not different brands of vinegar have different acidities. They all say that they are 5% acidity, but there is a range of values around this 5% that are allowed. You know that vinegar is just diluted acetic acid, so we can use a base like sodium hydroxide to neutralize it. If we titrate exact amounts and use phenolphthalein as an indicator we can record the volume of a known molarity of NaOH needed to neutralize the vinegar, and then use this value to calculate the molarity of the acetic acid. Let me show you how this works. I have ?M NaOH here and 0.1M HCl. If I start with 10ml of HCl in this Erlenmeyer flask, we can note many ml of NaOH I need to add to it in order to neutralize it, and use that volume to calculate the concentration of the NaOH. Let’s see how accurate the molarities of my solutions are. What color will phenolphthalein be in the NaOH? Pink What color would it be in something neutral or acidic? Clear So what I’m going to do is put my NaOH in the burette and add it slowly to the HCl until the solution turns from clear to pink. First I have to note my starting volume of NaOH, and then I can add it in a constant stream until I see the solution start to do turn pink at the point where the base is hitting the acid. (It can help to put a white paper underneath.) Once that happens I want to add the NaOH drop by drop until suddenly the whole solution will go clear. At that point I can note my ending volume and subtract the difference to get the volume of NaOH used. Of I plug this number back in to the equation leaving one of the other numbers out, I should be able to see if my molarities were accurate. In the lab you are going to put the vinegar in the flask with phenolphthalein and note when it turns pink as you add NaOH.