Periodic Trends – Full Lab Purpose: to graph different information of
advertisement

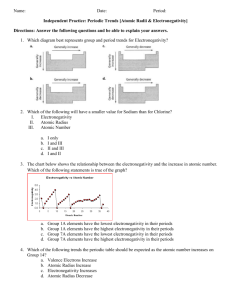
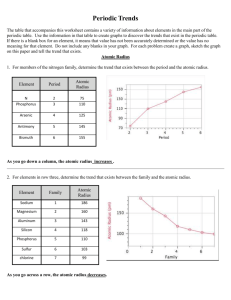
Periodic Trends – Full Lab Purpose: to graph different information of the representative elements to look for period and group trends. Procedure: 1. You will have 4 sections for this lab (atomic radius, ionic radius, ionization energy, and electronegativity). 2. Under each section you will have a data and a conclusion. 3. Use the provided graph paper to graph the trend vs. atomic number for periods 2-5. Atomic Radius: 1. Use the table below to create your graph of atomic radius vs. atomic number. Atomic Radius for Periods 2-5 in picometers Li 128 Na 166 K 203 Rb 220 Be 96 Mg 141 Ca 176 Sr 195 B 84 Al 121 Ga 122 In 142 C 76 Si 111 Ge 120 Sn 139 N 71 P 107 As 119 Sb 139 O 66 S 105 Se 120 Te 138 2. Remember to label each atom so you can tell groups from periods. 3. Answer the following questions based on your graph. a. Define atomic radius. b. Comment on the sizes of the atomic radii as you go across a period. c. Comment on the sizes of the atomic radii as you go down a group. d. Are there any patterns (trends) that you see for periods 2-5? e. Describe and explain the shape of each period’s portion of the graph. f. Are there any patterns (trends) that you see for each group? g. Describe and explain the shape of each group’s portion of the graph. F 57 Cl 102 Br 120 I 139 Ne 58 Ar 106 Kr 116 Xe 150 Ionic Radius: 1. Use the table below to create your graph of ionic radius vs. atomic number. Ionic Radius for Periods 2-5 in picometers Li 60 Na 95 K 133 Rb 148 Be 44 Mg 66 Ca 99 Sr 112 B 23 Al 51 Ga 62 In 81 C 15 Si 41 Ge 53 Sn 71 N 146 P 212 As 222 Sb 62 O 140 S 184 Se 198 Te 221 2. Remember to label each atom so you can tell groups from periods. 3. Answer the following questions based on your graph. a. Define ionic radius. b. Comment on the sizes of the ionic radii as you go across a period. c. Comment on the sizes of the ionic radii as you go down a group. d. Are there any patterns (trends) that you see for periods 2-5? e. Describe and explain the shape of each period’s portion of the graph. f. Are there any patterns (trends) that you see for each group? g. Describe and explain the shape of each group’s portion of the graph. h. How are the radii for the anions and cations different from their atomic radius? F 133 Cl 181 Br 196 I 220 Ne -Ar -Kr -Xe -- Ionization Energy: 1. Use the table below to create your graph of ionization energy vs. atomic number. First Ionization Energy for Periods 2-5 in kg/mol Li 520 Na 496 K 419 Rb 403 Be 900 Mg 738 Ca 590 Sr 550 B 801 Al 578 Ga 579 In 558 C 1087 Si 787 Ge 762 Sn 709 N 1402 P 1012 As 947 Sb 834 O 1314 S 1000 Se 941 Te 869 2. Remember to label each atom so you can tell groups from periods. 3. Answer the following questions based on your graph. a. Define first ionization energy. b. Comment on the sizes of the ionization energy as you go across a period. c. Comment on the sizes of the ionization energy as you go down a group. d. Are there any patterns (trends) that you see for periods 2-5? e. Describe and explain the shape of each period’s portion of the graph. f. Are there any patterns (trends) that you see for each group? g. Describe and explain the shape of each group’s portion of the graph. F 1681 Cl 1251 Br 1140 I 1008 Ne 2081 Ar 1521 Kr 1351 Xe 1170 Electronegativity: 1. Use the table below to create your graph of electronegativity vs. atomic number. Electronegativity for Periods 2-5 (no units used for this) Li 1.0 Na 0.9 K 0.8 Rb 0.8 Be 1.5 Mg 1.2 Ca 1.0 Sr 1.0 B 2.0 Al 1.5 Ga 1.6 In 1.7 C 2.5 Si 1.8 Ge 1.8 Sn 1.8 N 3.0 P 2.1 As 2.0 Sb 1.9 O 3.5 S 2.5 Se 2.4 Te 2.1 2. Remember to label each atom so you can tell groups from periods. 3. Answer the following questions based on your graph. a. Define electronegativity. b. Comment on the sizes of the electronegativity as you go across a period. c. Comment on the sizes of the electronegativity as you go down a group. d. Are there any patterns (trends) that you see for periods 2-5? e. Describe and explain the shape of each period’s portion of the graph. f. Are there any patterns (trends) that you see for each group? g. Describe and explain the shape of each group’s portion of the graph. F 4.0 Cl 3.0 Br 2.8 I 2.5 Ne -Ar -Kr -Xe --