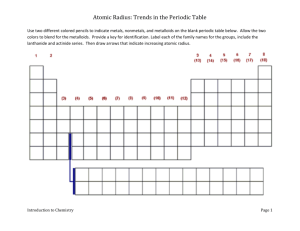
Trends in Atomic Radius Graphing Activity
Trends in Atomic Radius Graphing Activity
Name____________________________________________ Period_____ Date___________________
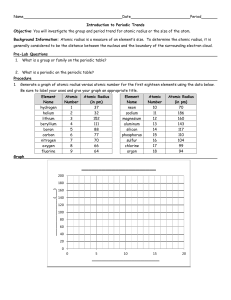
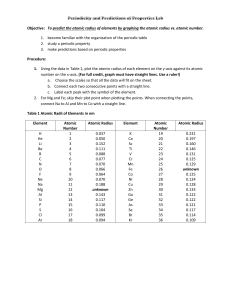
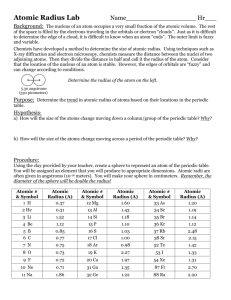
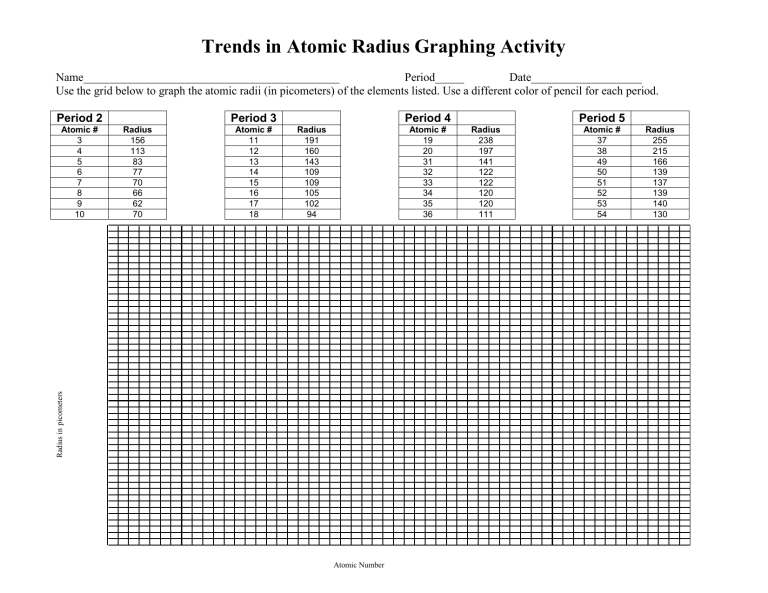
Use the grid below to graph the atomic radii (in picometers) of the elements listed. Use a different color of pencil for each period.
Period 2
Atomic #
6
7
8
9
3
4
5
10
Radius
156
113
83
77
70
66
62
70
Period 3
Atomic #
11
12
13
14
15
16
17
18
Radius
191
160
143
109
109
105
102
94
Period 4
Atomic #
19
20
31
32
33
34
35
36
Radius
238
197
141
122
122
120
120
111
Period 5
Atomic #
37
38
49
50
51
52
53
54
Radius
255
215
166
139
137
139
140
130
Atomic Number
Trends in Atomic Radius Graphing Activity
Analysis Questions:
1) Describe the trend shown in each period. Try using a phrase like “As you go across the period from left to right, the atomic radius...” (1 pt)
2) How is the graph DIFFERENT for each period (aside from the color of pencil or ink you used to draw the lines)? (1 pt)
3) Describe the trend shown as you go down a group. This trend is probably something you expected to see. (1 pt)
4) Which group contains the elements with the largest atomic radii? (1 pt)