Quiz2.docx
advertisement

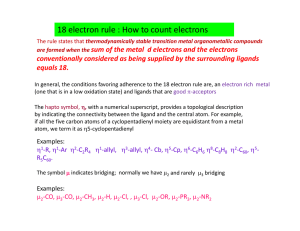
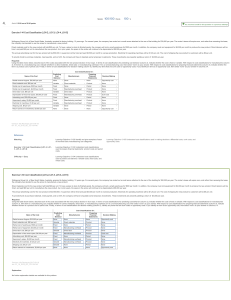
Name ____________________ CHEM 1004 Quiz #2 Spring 2011 Buckley 1. (3 points) Give the chemical symbol for the neutral element that corresponds to each of the following electron configurations. a. 1s22s22p4 ________________ b. 1s22s22p63s23p64s2 ______________________ c. 1s22s22p63s23p64s23d104p65s1 _____________________ 2. (3 points) Give the number of valence electrons for each of the elements in Question 1. a. _________ b. __________ c. ____________ 3. (3 points) For each of the elements in Question 1, state whether it is a metal or a nonmetal. a. ______________ b. ________________ c. _______________ 4. (3 points) For each of the electron configurations in Question 1, state every one of the classifications that applies from the list: alkali metal, alkaline earth metal, chalcogen, halogen, noble gas, main group element, transition element, inner transition element. Not all classifications will be used and some classifications may be used more than once. a. ________________________________________________________ b. ________________________________________________________ c. ________________________________________________________ 5. (9 points) Complete the following table. Species 75 33 34 16 90 38 Protons Neutrons Electrons As S 2 Sr 2 ←←←←←PLEASE TURN THE PAGE OVER→→→→→ 6. (2 points) Niels Bohr explained the line spectrum as shown below (not shown very well on a black and white document, but you get the idea). Briefly give his explanation as to the origin of the discrete lines seen in atomic spectra. You might consider things like why do we only see a few lines, what do they correspond to, and what new idea does this introduce to our understanding of the atom. ←←←←←PLEASE TURN THE PAGE OVER→→→→→