Study Guide for Chemistry Test – Electron configurations & Periodic
advertisement

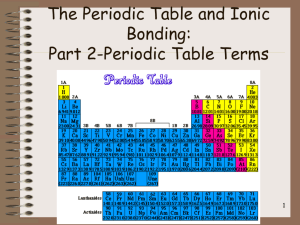
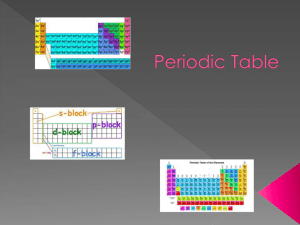
Study Guide for Chemistry Test –Electrons in Atoms & Periodic Table Sections in the book – 13.1 – 13.3, 5.4, 14.1 – 14.2 Words/Terms to know: Periodic table Halogens photons Mendeleev Alkali Metals ground state Periods Alkaline Earth Metals excited state Group or family Noble Gases flame test Representative elements energy level Transition metals quantum mechanical model Inner transition metals atomic orbitals Metals sublevels (subshells) Nonmetals Energy levels (energy shells) Metalloids or Semimetals electron configurations (rules to write them) s, p, d, f blocks electromagnetic radiation Electron configuration wavelength Valence electrons frequency Electron dot structures atomic emission spectrum Octet Rule continuous spectrum Good to review: Notes, worksheets, quiz on periodic table Pg 409 #10-12, 14, 18, 22, 24, 27 Pg 432 #20, 21, 23, 24 Test Set up – Multiple Choice, True/False, Diagram of Periodic Table that you will have to identify specific parts of it (groups and families and types of elements – metals, nonmetals, metalloids), Problems – calculate the wavelength, frequency or energy of light, electron configurations, determine valence electrons and write dot structures of elements, determine the trends using the periodic table Possible short answer questions – Determine the identity of an unknown element using chemical and physical properties Explain why metals lose electrons and why nonmetals gain electrons. Answers to review questions: Pg 409 #10-12, 14, 18, 22, 24, 27 10 Noble gases are found in group 18 and they are the least reactive elements, Representative elements are found in groups 1,2 and 13 – 18, the transition elements are found in groups 3 – 12, and the inner transition elements are found in the two rows below the periodic table 11 a) B 1s22s22p1 b) Mg 1s22s22p63s2 c) As 1s22s22p63s23p64s23d104p3 12 Na, Mg, and Cl are representative elements 14 This is because the outermost electron configuration tells us the block, the row and the number of elements over in the block that the element is located. For example [Ar] 4s2 tells us that the element is located in the s-block in row 4 and it is the second one over in the s-block making it the element Calcium 18 a) sodium b) strontium c) germanium d) selenium 22 a Sr, Mg, Be b) Cs, Ba, Bi c) Na, Al, S 24 a) Na b) S2- c) I - d) Al 27 a) F b) N c) Mg d) As Pg 432 #20, 21, 23, 24 20. Valence electrons are electrons that are in the highest energy level of an atom 21. Fluorine, Chlorine, Bromine, Iodine. They are in group 17, They all have 7 valence electrons .. .. . . 23. a) :Cl . b) :S . c) Al . d) Li .. . . 24. B/c the energy level are all full of electrons