Valence Electron configuration and the Periodic table
advertisement

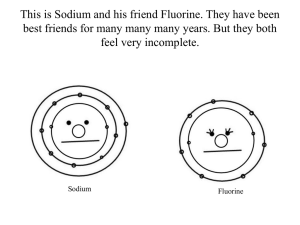
Valence Electron configuration and the Periodic table In chemistry, a valence electron is an electron that is associated with an atom, and that can participate in the formation of a chemical bond; in a single covalent bond, both atoms in the bond contribute one valence electron in order to form a shared pair. The presence of valence electrons can determine the element's chemical properties and whether it may bond with other elements: For a main group element, a valence electron can only be in the outermost electron shell. In a transition metal, a valence electron can also be in an inner shell. An atom with a closed shell of valence electrons (corresponding to an electron configuration s2p6) tends to be chemically inert. Atoms with one or two more valence electrons than are needed for a "closed" shell are highly reactive because the extra valence electrons are easily removed to form a positive ion. Atoms with one or two valence electrons fewer than are needed to form a closed shell are also highly reactive because of a tendency either to gain the missing valence electrons (thereby forming a negative ion), or to share valence electrons (thereby forming a covalent bond). Like an electron in an inner shell, a valence electron has the ability to absorb or release energy in the form of a photon. An energy gain can trigger an electron to move (jump) to an outer shell; this is known as atomic excitation. Or the electron can even break free from its associated atom's valence shell; this is ionization to form a positive ion. When an electron loses energy (thereby causing a photon to be emitted), then it can move to an inner shell which is not fully occupied. Valence energy levels correspond to the principal quantum numbers (n = 1, 2, 3, 4 ...) or are labeled alphabetically with letters used in the X-ray notation (K, L, M, …). Major Divisions of the Periodic Table The periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers, electron configurations, and chemical properties. Elements are presented in increasing atomic number. The main body of the table is a 18 × 7 grid. Elements with the same number of valence electrons are kept together in groups, such as the halogens and the noble gases. There are four distinct rectangular areas or blocks. The f-block is usually not included in the main table, but rather is floated below, as an inline f-block would often make the table impractically wide. Using periodic trends, the periodic table can help predict the properties of various elements and the relations between properties. It therefore provides a useful framework for analyzing chemical behavior and is widely used in chemistry and other sciences. Atomic Orbitals The electrons in the partially filled outermost shell (or shells) determine the chemical properties of the atom; it is called the valence shell. Each shell consists of one or more subshells, and each subshell consists of one or more atomic orbitals. The properties of an atom depend ultimately on the number of electrons in the various orbitals, and on the nuclear charge which determines the compactness of the orbitals. In order to relate the properties of the elements to their locations in the periodic table, it is often convenient to make use of a simplified view of the atom in which the nucleus is surrounded by one or more concentric spherical "shells," each of which consists of the highest-principal quantum number orbitals that contain at least one electron; these are sand p-orbitals and can include d- or f-orbitals, which is atom dependent. The shell model, as with any scientific model, is less a description of the world than a simplified way of looking at it that helps us to understand and correlate diverse phenomena. The organizational logic of the periodic table Starting at the top, follow the logic as the structure of the periodic table is broken down into functional divisions. We will look at several visualizations of the periodic table. First, however, it would be instructive to see how it is constructed from a logical viewpoint. The table today is the result of an ongoing effort of more than 100 years of observation, measurement, prediction and proof of the relationships of chemical and physical phenomena to electron configurations and charges. Periods 1, 2, & 3 Starting with simple elements, the first three rows of the periodic table, called Periods 1, 2 and 3, correspond to the n=1, n=2 and n=3 levels. Electron shell configurations of the first 18 elements The electron shell configurations of the first 18 elements in the periodic table. The corresponding energy levels (n) are listed in green numbers to the left. The number of outer-shell electrons is represented by the right-most digit in the group numbers.