Periodic table structure reflects electron configuration
advertisement

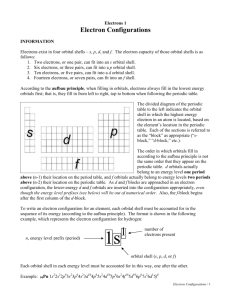
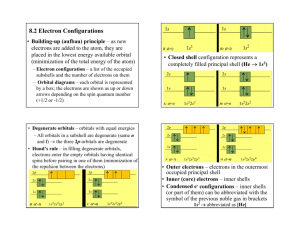
Dear students, do not miss between the Aufbau principle and order of orbitals in Periodic Table. Periodic table structure reflects electron configuration. The primary determinant of an element's chemical properties is its electron configuration, particularly the valence shell electrons. For instance, any atoms with four valence electrons occuping a p orbital will exhibit with some similarity. The type of orbital in which the atom's outermost electrons reside determines the "block" to which it belongs. The number of valence shell electrons determines which family, or group, the element belongs. The total number of electron shells an atom has determines the period to which it belongs. Each shell is divided into different subshells, which as atomic number increases are filled in roughly this order (the Aufbau principle): Subshell: S G F D P Period 1 2 3 4 5 6 7 8 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p 8s 5g 6f 7d 8p Hence the structure of the table. Since the outermost electrons determine chemical properties, those with the same number of valence electrons are grouped together. Progressing through a group from lightest element to heaviest element, the outer-shell electrons (those most readily accessible for participation in chemical reactions) are all in the same type of orbital, with a similar shape, but with increasingly higher energy and average distance from the nucleus. For instance, the outer-shell (or "valence") electrons of the first group, headed by hydrogen all have one electron in an s orbital. In hydrogen, that s orbital is in the lowest possible energy state of any atom, the first-shell orbital (and represented by hydrogen's position in the first period of the table). In francium, the heaviest element of the group, the outer-shell electron is in the seventh-shell orbital, significantly further out on average from the nucleus than those electrons filling all the shells below it in energy. As another example, both carbon and lead have four electrons in their outer shell orbitals. Note that as atomic number (i.e. charge on the atomic nucleus) increases, this leads to greater spin-orbit coupling between the nucleus and the electrons, reducing the validity of the quantum mechanical orbital approximation model, which considers each atomic orbital as a separate entity. Because of the importance of the outermost shell, the different regions of the periodic table are sometimes referred to as periodic table blocks, named according to the sub-shell in which the "last" electron resides, e.g. the s-block, the p-block, the d-block, etc. Orbitals in Periodic Table: The order in which the states are filled is as follows: