Lab 9 - Academic Computer Center
advertisement

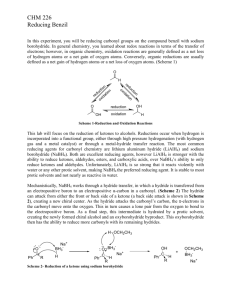
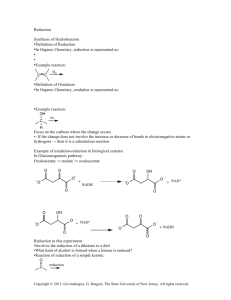
The Reduction of Benzil to Hydrobenzoin Introduction Figure 1 shows the reduction of a carbonyl group found in an aldehyde or ketone to an alcohol. The reaction requires two steps. In Step 1, Na+ and H- add to the carbonyl to make an intermediate salt. In the workup of Step 2, the salt is converted into an alcohol by protonation of the negative oxygen. The proton of Step 2 normally comes from a mineral acid such as HCl. O C NaBH4 H+ O- Na+ C H O C H H H2O Step2 Reduction of an Aldehyde or Ketone to an Alcohol by NaBH4 Step1 Figure 1. Reduction of a carbonyl group. Reduction involves the loss of oxygen and/or the addition of hydrogen atoms. Three common reducing agents are lithium hydride, LiH, lithium aluminum hydride, LiAlH4, and sodium borohydride, NaBH4. Notice that all three contain hydrogen in a negative or hydride form. A hydride ion, H-, is a nucleophile that readily adds to a carbonyl carbon, which is positive relative to the oxygen of the double bond. These three reducing agents all contain a metal; therefore, the intermediate product of the reaction is an ionic salt, which must be acidified to obtain the final organic covalent product. Figure 2 shows a simplified mechanism for the reduction of a carbonyl group by a hydride ion, H-, which comes from NaBH4. O C H- OC H+ O C H H H2O Step2 Mechanism of the Reduction of an Aldehyde or Ketone to an Alcohol Step1 H Figure 2. Mechanism of Reduction. Lab 9 1 The overall reduction of a carbonyl group to a hydroxyl group involves the addition of two H atoms. The first H atom comes from a hydride, H-, of NaBH4. The second comes from the workup of the reaction, which is normally conducted in aqueous acid. Sodium borohydride, NaBH4, is the mildest of the three hydride reagents and is easy to use in the lab, because it is soluble in water, methanol and ethanol and does not react with these solvents. Therefore, NaBH4 is the reagent of choice for reducing aldehydes and ketones. Lithium aluminum hydride, LiAlH4, is a stronger reducing agent than NaBH4, and LiAlH4 is used to reduce carboxylic acids, epoxides, esters, lactones, nitro groups, nitriles, azides, amides and acid chlorides. Lithium hydride, LiH, is a strong base as well as a reduction agent. For example, LiH deprotonates an alcohol to make a lithium alkoxide and hydrogen gas. Figure 3 shows reduction reactions that require LiAlH4; each requires an acidification step to convert the initially formed salt into a covalent organic compound. When two steps are shown on one reaction arrow, they are shown in parenthesis [i.e., (1) and (2)]. O CH3CH2CH2COCH2CH3 (1) LiAlH4 (2) H3O+ ethyl butanoate CH3CH2CH2CH2OH + HOCH2CH3 butanol ethanol two alcohols an ester OH O O (1) LiAlH4 OH + (2) H3O -valerolactone a lactone 1,5-pentanediol two alcohols Figure 3. Reduction of an ester and a lactone. Exceptionally strong reducing agents convert aldehydes and ketones into alkanes. Three reactions are particularly useful. They are the Clemmensen reduction, the Wolff-Kishner reduction, and the Raney nickel reduction reactions. These three reactions may be conducted in acidic, basic and neutral solutions, respectively. The Clemmensen reagents are zinc amalgam [Zn(Hg)] in hydrochloric acid. The Wolff-Kishner reagents are hydrazine (H2NNH2) in base. The use of Raney nickel involves first converting the carbonyl group into a cyclic thioacetal and then reducing the sulfur compound with Raney nickel, a form of Lab 9 2 nickel that contains elemental hydrogen. Examples of reductions with these reducing agents are shown in Figure 4. O CCH2CH3 Zn(Hg) CH2CH2CH3 HCl reflux Clemmensen Reduction O CH H2NNH2 CH3 KOH DMSO (solvent) Wolff-Kishner Reduction H O S SH HS S H Ni(R) (H2) H+ Raney Nickel Reduction Figure 4. Reduction reactions of carbonyl groups to alkanes. The Experiment In this experiment, the diketone known by the common name benzil is the organic substrate. Sodium borohydride reduces both carbonyl groups in benzil, and acidification produces a diol or two secondary alcohols. See Figure 5. Lab 9 3 HO O O C C benzil * H HO (1) NaBH4 (2) H2O (H+) H HO * Ph Ph meso * H HO Ph HO Ph * H 1S,2S * Ph HO H H * Ph 1R,2R hydrobenzoin Figure 5. The sodium borohydride reduction of benzil (Ph = phenyl). The product of the reaction contains two chirality centers shown by asterisks (*). The maximum number of stereoisomers for a compound with two chirality centers is four (22). However, when the two centers contain the exact same four groups, a meso form is possible, and the total number of stereoisomers is three. Thus, the reduction of benzil produces two chirality centers but only three stereoisomers. Figure 5 shows the structures of the three stereoisomers. Though all three stereoisomers are produced, the meso form is the major product of this reduction reaction. Benzil is a yellow solid and hydrobenzoin is white; therefore, the reduction reaction is evident by the color change. Reduction of an aldehyde or ketone carbonyl group with NaBH4 is a general, two-step, reaction that is frequently seen in reaction schemes on standardized examinations. Lab 9 4 Procedure Reminder: Record all data directly in your notebook and turn in your data sheet with your lab report! 1. Add approximately 0.5 g of benzil (yellow solid) to a 50-mL Erlenmeyer flask and record the exact mass of the benzil in your notebook. 2. Add 5 mL of 95% ethanol to the Erlenmeyer flask that contains the benzil. 3. Cool the Erlenmeyer flask by rotating it under a stream of tap water. 4. Weigh 0.1-g NaBH4 on a waxed paper and transfer the white solid to the Erlenmeyer flask that contains benzil. 5. Gently swirl the Erlenmeyer flask until the benzil dissolves and the yellow color disappears. The exothermic reaction is over within three minutes. 6. After the yellow color is gone, warm the flask on a hot plate until the solution begins to boil. 7. As soon as the solution boils, carefully move the hot Erlenmeyer flask from the hot plate to the bench top, using hot hands or tongs to handle the hot glassware. 8. Allow the flask to cool on the bench top until crystals no longer appear to form. Then, place the Erlenmeyer flask in an ice and water bath for an additional five minutes. 9. Collect the crystals (mp 136-137 oC) on a Bűckner or Hirsh funnel, show the crystals to the instructor, and proceed as directed by the instructor. 10. Clean all glassware and return it to the same location where you found it. Clean your bench top and return all equipment to its storage location. Lab 9 5 Reduction Last name_____________________________, First ____________________________ 1. Calculate the oxidation number for the starred carbon in each of the following structures. O OH * * * 2. Why do reduction reactions of aldehydes and ketones with NaBH4 and LiAlH4 require an acidification step? 3. Show structures in the sawhorse projection for all stereochemical products for the reduction of the diketone shown below. O O (1) NaBH4 C C CH3 (2) H+ 4. What term describes the stereochemical relationship between (1R,2R)hydrobenzoin and (1S,2S)-hydrobenzoin? _____________________ What term describes the stereochemical relationship between meso-hydrobenzoin and (1R,2R)-hydrobenzoin? _________________________________ Show the major organic product(s) for each of the following equations. 5. H2NNH2/KOH CH3CH2CH2CHO (DMSO) 6. O Zn(Hg)/HCl (reflux) Lab 9 6 7. O O (1) LiAlH4 (2) H+ 8. O O (1) xs NaBH4 (2) H+ 9. Show the mechanism for the sodium borohydride reduction of acetaldehyde to ethanol. 10. Draw a complete structure for each of the following families and label each structure accordingly (aldehyde, ketone, ester, and lactone). No credit is given for partial (incomplete) structures. Lab 9 7