C=O Reduction Experiment
advertisement

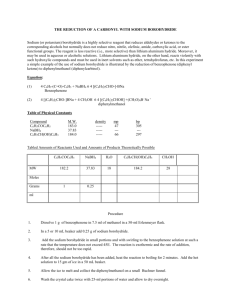
THE REDUCTION OF A CARBONYL WITH SODIUM BOROHYDRIDE pp 495-498 Sodium (or potassium) borohydride is a highly selective reagent that reduces aldehydes or ketones to the corresponding alcohols but normally does not reduce nitro, nitrile, olefinic, amide, carboxylic acid, or ester functional groups. The reagent is less reactive (i.e., more selective) than lithium aluminum hydride. Moreover, it may be used in aqueous or alcoholic solutions. Lithium aluminum hydride, on the other hand, reacts violently with such hydroxylic compounds and must be used in inert solvents such as ether, tetrahydrofuran, etc. In this experiment a simple example of the use of sodium borohydride is illustrated by the reduction of benzophenone (diphenyl ketone) to diphenylmethanol (diphenylcarbinol). Equation: (1) 4 C6H5-(C=O)-C6H5 + NaBH4 4 [(C6H5)2CHO-]-BNa Benzophenone (2) 4 [(C6H5)2CHO-]BNa + HCl + 3H2O 4 [(C6H5)2CHOH] + H3BO3 + NaCl diphenylmethanol Table of Physical Constants Compound C6H5COC6H5 NaBH4 C6H5CH(OH)C6H5 M.W. 183.0 37.83 184.0 density ------------- mp 47 --66 bp 305 --297 Tabled Amounts of Reactants Used and Amounts of Products Theoretically Possible MW C6H5COC6H5 NaBH4 HCl H2 O C6H5CH(OH)C6H5 H3BO3 NaCl 182.2 37.83 36.5 18 184.2 61.8 58.5 2.2 0.5 Moles Grams ml Procedure 1. Dissolve 2.2 g of benzophenone in 15 ml of methanol in a 150-ml beaker. 2. In a smaller beaker prepare a solution of 0.5 g of sodium borohydride in 7.5 ml of cold water. Add the aqueous sodium borohydride solution in small portions and with stirring to the benzophenone solution at such a rate that the temperature does not exceed 45. The reaction is exothermic and the rate of addition, therefore, should not be too rapid. 3. After all the sodium borohydride solution has been added, continue to stir the reaction mixture for approximately 15 minutes. A white gum results which is the product of eq. 1 above. IF your product oils or is not gummy, leave the flask in your desk until the next lab period before proceding to step 4. 4. Decompose the excess sodium borohydride and hydrolyze the white gum by adding the crystalline slurry slowly and with stirring to a mixture of 50 g of crushed ice and water and 10 ml of concentrated hydrochloric acid in a 400-ml beaker. Note: The decomposition of sodium borohydride is a vigorous reaction and hydrogen is liberated rapidly. Unless a large beaker is used, the froth which results from the decomposition cannot be confined. 5. Collect the diphenylmethanol on the Buchner funnel. 6. Wash the crystal cake twice with 25-ml portions of water and allow to dry overnight. 7. At the beginning of the next lab period, weigh the product, and determine its melting point. Yield about 2.2 g (95%). Include in your notebook and in your Lab Report, also calculate the % yield. 8. Use this product in the alcohol classification assignment. Explanation of the Procedure STEP 1 Sodium borohydride reacts with water to form H 2 gas so, instead of weighing, which exposes the reducing agent to the water in the air for a significant length of time, you quickly dump a rounded teaspoonful into the methanol. The reaction with water is H2O + Na+BH4- ---> Na+(BH3OH)- + H2 (gas) STEPS 2 & 3 The reaction between sodium borohydride and benzophenone is kept below 50 oC to minimize the side reaction of sodium borohydride with the solvent methanol. The side reaction is CH3OH + Na+BH4- ----> (CH3OBH3)-Na+ + H2 and can go to (CH3O)4B-Na+ + 4H2 if all of the NaBH4 reacts with the solvent. The mechanism of the main reaction involves hydride, H-, leaving NaBH4 + going to the benzophenone, see pg 496 of lab text. The mechanism is C6H5 H C=O + H-B-H H C6H5 C6H5 Na+ -----> H-C-OBH3 Na+ C6H5 The reaction continues until all of the H on BH4 have been replaced by H C6H5-C-C6H5 O C6H5 C6H5 H 3 C=O reacting with BH3- ---> HC-O-B-O-C-C6H5 C6H5 O C6H5 O C6H5 H5C6-C-C6H5 C6H5-CH H C6H5 which is the white gum produced in equation (1) shown at the beginning of the experiment. STEP 4 The borohydride salt of diphenylmethanol is hydrolyzed in two steps by the aqueous HCl. Reaction (a) is H C6H5CC6H5 C6H5 O H OH OH HC-O- B-O-C-CH6H5 + 4 H2O ---> HO-B-OH Na+ + 4C6H5-C-C6H5 C6H5 O C6H5 OH H C6H5-C-C6H5 H OH HO-B-OH OH OH Na+ + HCl ----> B + H2O + NaCl HO OH Lab Report. Follow outline on pg 5 and the instructions at step 7 of this experiment. HOMEWORK 1. Determine the molar ratio of NaBH4 to benzophenone that you ACTUALLY used in the experiment. why do you think that it was necessary to use a greater molar ratio than the equations at the beginning indicate? 2. After the reaction between the sodium borohydride and the carbonyl compound is complete, the reaction mixture is treated with water to produce the desired secondary alcohol. Explain this reaction by indicating the source of the hydrogen that ends up on oxygen. 3. Although sodium borohydride is fairly unreactive toward methanol, the addition of a mineral acid to this solution results in the rapid destruction of NaBH4. Explain. (Think in terms of the reactivity of Zno with acetic acid compared to that with HCl.) 4. Give the structure of the white precipitate formed in the reaction of benzophenone with sodium borohydride, and write an equation for its reaction with water. 5. What reagents will be used for the conversion of benzophenone to diphenylmethanol. Determine the limiting reagent and the theoretical yield of product. Show your work. 6. What types of carbonyl compounds could be used to prepare a primary or secondary alcohol? Could a tertiary alcohol be prepared by the reduction of a carbonyl compound ? Explain.