Chap 3.6 - 3.7
advertisement

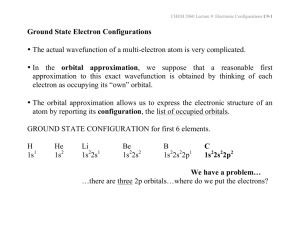
3.6: ATOMIC STRUCTURE AND THE PERIODIC TABLE CREATING ENERGY-LEVEL DIAGRAMS FOR ATOMS See Fig 2, p. 187.(Draw the sample diagram into your notebooks) Diagram: DISTRIBUTION OF ELECTRONS IN ORBITALS Begin with Hydrogen → one proton and one electron in the Is Orbital. Then each time we add a proton to the nucleus, we also add an electron to an available orbital. Rules: 1. Electrons occupy the lowest energy orbital of the lowest energy level first. (The Aufbau Principle) 2. No electron pairing takes place in the p,d or f orbital until each orbital of a given set contains one electron (Hund’s Rule) 3. No orbital can contain more than two electrons. (Pauli Exclusion principle) states that no two electrons in the same atom may have identical values for all four quantum numbers. creating energy-level diagrams for anions (negative charge ions) The energy-level diagrams for anions, are done using the same method as for atoms. But you need to add the extra electrons corresponding to the ionic charge to the total number of electron before distribution of the electrons on the orbitals (See p190) creating energy level diagram for cations (positive charged ions) This is different from anions. You must draw the energy level diagram for the corresponding neutral atom first, and then remove the number of electrons (corresponding to the charge) from the orbitals with the highest principal quantum number, n. Note: This may not be the highest –energy electron (See p.190) ELECTRON CONFIGURATION The order of increasing energy, the orbital sequence. 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p…… ↑ (4s has less energy than 3d.) Valence electrons e.g. Group 1 – valence of one (Oxidation Number Group 2 – valence of two PERIODICITY OF OUTER ENERGY-LEVEL ELECTRON CONFIGURATION Atoms of all elements in a given group or family of the Periodic table have the same electron population and orbital configuration in their outer energy levels. Each group is composed of elements having similar properties but each group has properties, which differ from those of the other groups. SUMMARY GROUP A ALKAL1 METALS 2s1 Lithium Li [He] Sodium Na [Ne] 3s1 Potassium k [Ar] Rubidium Rb [Kr] 5s1 Cesium Cs [Xe] 6s1 GROUP II A 4s1 VERY REACTIVE METALS ALKALINE – EARTH METALS Beryllium Be [He] 2s2 Magnesium Mg [Ne] 3s2 Calcium Ca [Ar] 4s2 Strontium Sr [Kr] 5s2 GROUP III B IV B VB VI B VII B VIII B Transition Elements filling the d orbital block IB IIB GROUP II A Boron B [He] 2s2 2p1 Aluminum Al [Ne] 3s2 3p1 GROUP IV A Carbon C[He] 2s2 2p2 Silicon Si [Ne] 3s2 3p2 GROUP V A Nitrogen N[He] 2s2 2p3 Phosphorus p [Ne] 3s2 3p3 GROUP VI A P-Orbital block Oxygen [He] 2s2 2p4 Sulphur [Ne] 3s2 2p4 GROUP V11 A Fluorine [He] 2s2 2p5 Chlorine [Ne] 3s2 3p5 Bromine [Ar] 4s2 4p5 Iodine [Kr] 5s2 5p5 GROUP O NOBLE GAS (Highly stable) Helium 1s2 Neon 2s3 2p6 Argon 3s2 3p6 Krypton 4s2 4p6 Xenon 5s2 5p6 5s2 6p6 Radon f-Orbital block n =4 n =5 n =6 n=4 4s 3d n=5 4p 5s 4d n=6 5p 6s 4f n =7 5d 6p 7s 5f Lanthanide series Actinide series Inner Transition Elements They are in the f-orbital block IONIC CHARGE FORMATION The electrons in the highest or outermost energy level are the ones generally involved in chemical reactions. Valence electrons e.g. Group 1 – valence of one (Oxidation Number) Group 2 – valence of two The formation of ionic charges can be explained with the following: Zinc ion: The short hand form of the electron configuration for the atom is [Ar] 4s2 3d10. This shows 12 outer electrons. In forming the ion of Zn2+, the two electrons are removed from the 4s rather than the 3d10 which is more stable because of the electron clouds. Then, a relatively stable state, like atom with filled sub-shell is: Zn2+: [Ar] 3d10 ANOMALOUS ELECTRON CONFIGURATIONS The rules for electron Configuration as described above may not work for all of the elements. This is to ensure stability. Following the rules, we would expect the following configurations: Cr: [Ar] 4s2 3d4 Cu: [Ar] 4s2 3d9 However, the actual electron configurations, determined experimentally, are: Cr: [Ar] 4s1 3d5 Cu: [Ar] 4s1 3d10 Corresponding diagrams would be: Cr: [Ar] Predicted (↑↓) (↑) (↓) (↑) (↓) ( ) Cu: [Ar} (↑↓) (↑↓) (↑↓) (↑↓) (↑↓) (↑) 4s 3d Actual Cr: [Ar] (↑) Cu: [Ar] (↑) 4s (↑) (↑) (↑) (↑) (↑) (↑↓) (↑↓) (↑↓) (↑↓) (↑↓) 3d Note: For chromium, an electron is “borrowed” from the 4s sub-shell to give to 3d subshell that is half-filled For Copper, same arrangement. A similar thing happens with silver and gold Ag: [Kr] 5s1 4d10 Au: [Xe] 6s1 5d10 4f14 The borrowing of electrons also occurs in some of the ions formed by the transition elements. 3.7: Wave Mechanics and orbitals: The German physicist, Werner Heisenberg described the limitations of our ability to measure both a particle’s velocity and its position at the same instant. This is called Heisenberg’ uncertainty principle. This is particularly true for small particles such as electrons. This led to two ideas: Electron Cloud: Electron behaves as if it were spread out around the nucleus. This relates probability to amplitude or intensity Electron Probability Density: This relates to how much of electron’s charge is packed into a given volume. Because of the wave nature, the electron (and its charge) is spread out around the nucleus. The electron probability density for a 1s orbital of hydrogen atom shows a spherical shape. All of the s-orbitals are spherical. Their sizes increase with increasing size of n. A p-orbitals consists of three orbitals whose directions lie at 900 to each other along the axes of an imaginary xyz coordinate system. The p-orbital concentrated along the x-axis is called px , and so forth. Their size also increases with increasing size of n.