Section 1A
advertisement

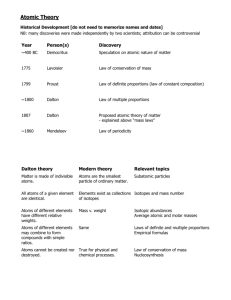
Section 1A Section 1A Structure of the Atom / Page 1 Structure of the Atom Atoms and Sub-atomic Particles One of the oldest ideas in science is that matter can be divided and further divided until the smallest possible particles of matter are obtained. This idea was put forward by the Greek philosopher, Dermocritus, in 400 B.C. He called the particles atoms (Greek: atomos, indivisible). In 1808, the British chemist John Dalton, formulated his Atomic Theory. He postulated that all matter consists of atoms, minute particles which cannot be created, destroyed or split. He theorized that all the atoms of an element are identical in every aspect, for example, their size and mass. These particles were indivisible and remained unchanged during a chemical reaction. The idea of indivisible atom was shown to be wrong by the works of a number of scientists in the late nineteenth century. The electron was the first sub-atomic particle to be identified. In 1897, Sir Joseph John Thomson discovered the electron through his work on cathode rays. He also determined the charge-to-mass (e/m) ratio of electron. In 1909, Milikan, through his famous oil-drop experiment, determined the charge e on an electron. Combined with Thomson's value for e/m ratio, it was then possible to calculate the mass m of an electron. Nowadays, the accepted values for these are e me = = 1.602 x 10 9.110 x 10 -19 -28 C g The Nucleus - Rutherford's Scattering Experiment In 1909, Lord Rutherford and his colleagues, Geiger and Marsden, performed the goldfoil scattering experiment. The Scattering Experiment Fast-moving alpha particles were directed to hit a piece of gold foil which was only a few hundred atoms thick. Result: 1. Most of the alpha particles passed through without changing direction. 2. A few were deflected through large angles. 3. Some even rebounded back. to vacuum pump thin gold foil movable fluorescent screen lead shield moveable microscope alpha particle source deflected beam of alpha particles rebounded beam of alpha particles Section 1A Structure of the Atom / Page 2 Explanation: Rutherford proposed that an atom consisted of a central, minute nucleus where the mass and positive charges of the whole atom were concentrated; the electrons occupied the remaining space in the atom, revolving around the nucleus. alpha particles gold atoms Summary i] ii] iii] of Rutherford's hypothesis: In the centre of the atom is a very small dense region called the nucleus. The electrons move around the nucleus at great speed. The number of electrons equals the number of protons. Most of the atom is empty space. The neutron was predicted by Rutherford in 1920 to account for the difference between atomic mass and atomic number. It was finally detected experimentally in 1932 by Sir James Chadwick. Therefore, an atom was found to consist of three fundamental sub-atomic particles, these are electrons which are negatively charged, protons which are positively charged and neutrons which are uncharged. Particle Relative mass Charge Proton 1 +1 Neutron 1 0 1 1836 Electron -1 Radioactivity A number of elements have atoms which are unstable and split up to form smaller atoms. This process is called radioactive decay. Three types of radiation are given off by radioactive substances: i) -particles: These are helium nuclei (He2+) in nature, i.e. positively charged. They have the lowest penetrating power. ii) -particles: These are electrons, i.e. negatively charged. Their penetrating power is higher than that of -particles. iii) -rays These are uncharged rays, similar to able to pass through 0.1 m of metal. X-rays. They have high penetrating power, being Equations for nuclear reactions A The symbol is used to represent the nuclide X with atomic number Z and mass ZX number A. The symbols used to represent the various types of radiation or particles are listed below: Particles / Radiation alpha particle Symbols 4 2 He or beta particle 0 -1 proton 1 1 e or p or 11 H neutron 1 0 gamma radiation n when Mass no. (A) Atomic no.(Z) Mass no. (A) Atomic no.(Z) Mass no. (A) Atomic no.(Z) Mass no. (A) Atomic no.(Z) Mass no. (A) Atomic no.(Z) emitted ____ by ____ . ____ by ____ . _______________ . ____ by ____ . ____ by ____ . ____ by ____ . ____ by ____ . ______________ . ______________ . ______________ . Section 1A Example: Structure of the Atom / Page 3 State what type of emission occurs at each of the following stages in the following decay chain: 228 88 ( a) ( b) (c ) Ra 228 228 224 89 Ac 90Th 88Ra (a) (b) (c) . __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ 1. Q P - + R S The above diagram shows how alpha and beta particles and gamma rays, emitted from a radioactive source, S, behave in an electric field. a) Use the information on the diagram to identify the type of emission present at Q, and R. __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ P, b) By what other means could a similar pattern of deflection of the three types of radiation be caused. __________________________________________________________________ Uses of Radioactivity i) Leak detection: A radioactive source is introduced into systems like storage tanks and buried pipelines, and a detector is used to locate the position of leakage. ii) Radiotherapy: -rays is used in cancer treatment. iii) Carbon-14 dating: By comparing the concentration of carbon-14 in the archaeological(考古學的) specimen and similar materials at present time, the age of the specimen can be estimated. iv) Nuclear power: Nuclear reactions result in the release of a vast quantity of energy, which is used to generate electricity. v) As tracers: Radioactive isotopes can be used as tracers to study metabolism(新陳代謝) in living organisms. . 2. a) b) 234 An isotope of uranium is represented by the symbol 92 U . What is the significance of the two numbers ? __________________________________________________________________ __________________________________________________________________ Identify A, B, C, D and E 234 230 i) A + 92 U 90Th ii) 239 92 U 235 92 iii) U B + 239 93 C + in the following equations: Np 235 92 iv) 238 92 U + 2 1 H 239 92 v) 235 92 U + 1 0 n 95 42 U U + D Mo + 139 57 La + 2 1 0 n + 7E Section 1A Structure of the Atom / Page 4 The Mass of an Atom Atomic Number This is the number of protons in the nucleus of an atom. It is given the symbol represents: i] the number of protons in the nucleus; ii] the number of electrons in a neutral atoms; and iii] ordinal number in the periodic table. Number This is the number of protons and neutrons in a nucleus. It is given the symbol This is always a whole number (c.f. atomic mass). Z . It Mass A . Nuclides This is any nuclear species of given mass number (A) and atomic number (Z), for example: 16 12 ; 6C . 8O Isotopes These are atoms of an elements which differ only in the number of neutrons they contain. They thus have the same atomic number but different mass number. 12 14 Examples: and 6 C 6C Isotopes Abundance Most elements consist of mixtures of isotopes. The abundance of each in the mixture is called its isotopic abundance. For example, silicon occurs in naturally occurring compounds as 29 30 92.28% 28 Si ; 4.67% Si and 3.05% Si. _____________________________________________________________________________________ . 3. The table below shows the mass number and number of neutrons in the nucleus, for four atoms, W, X, Y and Z. Mass number Neutrons in nucleus W 36 18 X 39 20 Y 40 21 Z 40 22 a) Write down the atomic numbers of the four atoms. __________________________________________________________________ __________________________________________________________________ b) Which of the four atoms are isotopes of the same element ? __________________________________________________________________ _____________________________________________________________________________________ Relative Isotopic Mass This is the ratio of the mass of a particular atom to 12 C. nuclide That is , Relative isotopic mass= 1 12 of the mass of the atom of the mass of a partic ular atom 12 1 C 12 mass of n uclide Relative Atomic Mass ( Ar ) This is the weighted average of the relative isotopic masses of an element. It is calculated by multiplying the relative isotopic mass of each isotope by its fractional abundance and adding all these values together. 35 37 Example 1: Chlorine consists of 2 natural isotopes, Cl and Cl, with percentage abundance of 75.4% and 24.6% respectively. Calculate the atomic mass of chlorine. Section 1A . 4. Neon in the air contains mass of neon. (20.2) 90% of 20 Ne and 10% Structure of the Atom of 22 / Page 5 Ne. Calculate the atomic Determination of Atomic Mass by Mass Spectrometer negatively charged accelerating grids electromagnet vaporization chamber ionization chamber to vacuum pump detector There are FIVE main operations performed by the mass spectrometer: 1] The sample of the material to be analysed, which may be an element or a compound, is injected and heated to vapourized in the vaporization chamber. The vaporized sample then passes into the ionization chamber. 2] Electrons are knocked out from the atoms of the element by bombardment with highenergy electrons produced by the hot-filament (thermionic emission). This cause ionization of the atomic sample and result in the production of positively charged ions which are mainly singly charged. X (g) + e X + (g) + 2 e fast slow 3] These positively charged ions are then acted upon by a 'velocity selector' (an electric field and magnetic field applied perpendicular to the pathway of the ions) which select those ions with the same velocity (though may differ in mass) to enter the circular chamber. 4] They were then deflected along a circular path by another known magnetic field applied perpendicular to the direction of movement of the ions. The lighter the positive ions, the greater is the deflection. Hence, at a given electric and magnetic field strength, only ions of a particular mass/charge ratio can hit the ion detector. 5] By varying the accelerating electric field or the deflecting magnetic field, ions of any mass/charge ratio can be brought to the ion detector. A mass spectrum can then be traced out by a recorder. Also, the relative abundance of each isotopes can be found from the relative magnitude of the signal (current) produced in the detector. In the mass spectrum of an element, the peaks can give information about various isotopes of the element. Whereas in the mass spectrum of a compound, the peak with the highest m/e ratio will most likely correspond to the ‘molecular ion’, i.e. the molecule which has lost only a single electron. In most mass spectra, the values of the m/e ratio can be converted to the relative masses of the particles if the charges on the ions are taken to be one. Therefore from the measurement of the mass spectrometer, two data about the sample element can be obtained: [i] the isotopic masses of each isotope ; and [ii] the relative abundance of each isotopes in the sample. _____________________________________________________________________________________ Example: Section 1A Structure of the Atom The following diagram is the mass spectrum of the element lead. / Page 6 de te ctor r e adi ng 204 206 208 (i) Give a full interpretation of this trace. __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ (ii) Calculate the atomic mass of lead (correct to 4 sig. figures ). . 5. The mass spectrum of neon consists of three lines corresponding to mass/charge ratios of 20, 21 and 22 with relative intensities of 0.910; 0.0026; 0.088 respectively. Explain the significance of these data and, hence, calculate the relative atomic mass of neon. 6. The isotopic composition of the gas radon was investigated using a mass spectrometer, part of which is shown in Fig. 4 below. magnetic field perpendicular to plane of diagram Y X detector a) Radon has two isotopes, i) 222 86 R n and 220 86 Rn. Write the formulae of the two singly-charged ions that would form in the instrument. ii) State which ion will follow the path marked X on the diagram. __________________________________________________________________ -1 b) Mention two adjustments that could be made to the instrument to bring the ions from Y on to the detector. __________________________________________________________________ __________________________________________________________________ c) If Rn ions were to form in the instrument, would you expect them to be deflected less than or more than the ions at X and Y ? __________________________________________________________________ __________________________________________________________________ 2+ Give an account of the use of a mass spectrometer for determining the relative masses of particles. (6marks) (902A3(a)) ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ Section 1A Structure of the Atom ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ 2 Name the particles charge and mass. 229 i) 90Th ii) 225 88 X and X + Y 225 88 / Page 7 in the following nuclear reactions, and give their X Ra is 225 Y + 89 Ac Y is Compare the action of a magnetic field upon the paths of Ra X and Y. (4 marks) (93IA1(a)) ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ Answer 1. to Exercise (902A3a) The modern method of the mass spectrometer. determining relative atomic B 4 sample the sample charged ions( 21 element) are ions travel along - magnetic into uses @ @ 1 2 1 2 marks marks is bombarded by electrons( 21 ) to form positively ). ions focused 2. (an particles magnetic field - the B. - the the parts explain electron beam - of ion detector electric field A masses accelerated( 21 ) field the until is ion - by the electric deflected( 21 ) adjusted( 21 ) detector( 21 so by that field( 21 ) the ions between magnetic of a plates A and mass are field( 21 ). particular ). - knowing the magnetic field strength , accelerating voltage of the path , the atomic mass can be calculated.(1) and - ( m / e ) the mass spectrometer ionized species.(1) responds to the mass - to - charge the radius of the (931A1a) (i) (ii) X Y is 4 2 is 0 1 He / helium nucleus / - particles e / fast moving electron / - particles The path of and - particles are deflected / but they are deflected in opposite directions (1). or x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x particle x or X x x x - particle or Y bent (1) direction (1) (1) (1) bent (1) by a magnetic field