Early Models of the Atom
advertisement

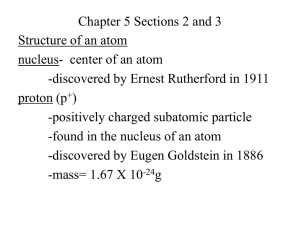
EARLY MODELS OF THE ATOM Powerpoint Templates Page 1 WHAT’S THE MATTER? •Ancient civilizations have wondered what made up the stuff around them. •Some early ideas included natural elements like earth, air, fire and water. (Empedocles) •The Greek philosopher, Democritus, suggested that matter could be cut down into smaller pieces until you reached a single indivisible particle which he called the atom. •As history moved along, others would experiment and observe to expand our knowledge of the structure of the atom. Powerpoint Templates Page 2 DALTON’S ATOMIC THEORY • John Dalton was a teacher that came up with his theory of atomic structure based on the work of many scientists of his era. • Dalton’s theory (1803) included the following: – Matter consists of definite particles called atoms. – Each element has its own type of atom. – Atoms of different elements have different properties. – Atoms of two or more different elements can combine in specific ratios to form compounds. – Atoms cannot be created or destroyed or broken up in a chemical change. • Future scientists would build upon Dalton’s work to help arrive at our current understanding of atomic structure. Powerpoint Templates Page 3 THE SUBATOMIC PARTICLES • The next scientist to assist in our understanding of atomic structure was J.J. Thomson in 1897. • Thomson used advances in technology to arrive at his famous raisin bun model of the atom. • He said atoms were like raisin buns in that you had a positively charged sphere with negatively charged particles called electrons – the “raisins” – scattered throughout. • This is kind of accurate if you think about what you already know about the atom. Powerpoint Templates Page 4 RAISIN BUN 2.0 • In 1911, Ernest Rutherford conducted experiments that furthered our understanding of atomic structure. • He concluded that the atom had a nucleus that held positive charges, which he named protons. • This nucleus was surrounded by mostly empty space which had negatively charged particles within it. • James Chadwick would suggest the existence of neutrons within the nucleus of the atom in 1932. The neutrons had the same mass as a proton but did not carry a charge. Powerpoint Templates Page 5 ATOMIC STRUCTURE Powerpoint Templates Page 6 THAT’S ALL FOLKS! Powerpoint Templates Page 7