Chapter 18 Review
advertisement

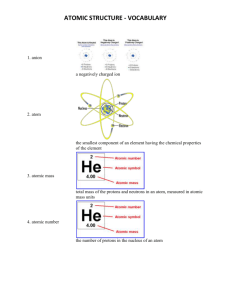
This scientist published a detailed atomic theory in 1808 based on evidence he gathered through experiments with gases. His atomic theory laid the groundwork for later atomic models. John Dalton British physicist Joseph John Thomson was the first to identify the: electron A common isotope of Carbon has a mass number of 13. The total number of protons in the nucleus would be: 6 The ____ is one kind of particle that makes up the atom and carries a positive charge. The neutron makes up for “missing mass” and is found in the nucleus. The third kind of particle carries a ____ charge and has almost no mass. Proton Negative Atoms with the same atomic number but different atomic mass are called: isotopes A – Atomic Number B – Chemical Symbol C – Name of Element D – Atomic mass Fireworks contain different elements in them for displaying different colors. The different colors occur because: atoms of various elements emit light at different frequencies Elements in the same column on the periodic table have similar chemical reactivity because: they have the same number of valence shell electrons How many energy levels are filled in a krypton atom? Four A – Proton B – Neutron C – Nucleus D – Energy Level E -- Electron **** A chemical symbol represents the _______________ of an element. Name How many protons are in the atom of the element above? 7 A certain atom has 26 protons, 26 electrons, and 30 neutrons. Its mass number is ____. 56 Describe where the electrons are in the atom, where they have the least energy, and where they have the most energy. Electrons go around the nucleus in a cloud. Energy is lowest close to the nucleus, and highest away from the nucleus. Use the periodic table to find the name, atomic number, and the average atomic mass of the following elements: O, S. O, oxygen, 8, 15.999 S, sulfur, 16, 32.066 Who developed the first periodic table? Mendeleev