PHYS 212-071
advertisement

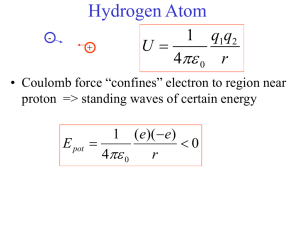
PHYS 212-071 HW # 5 Solutions (Numbers refer to 2nd Edition) 18. A hydrogen atom initially in its ground state ( n 1) absorbs a photon and ends up in the state for which n 3 . (a) What is the energy of the absorbed photon? (b) If the atom returns to its ground state, what photon energies could the atom emit? (a) E1 13.6eV , E3 13.6eV 1.5eV E Photon E3 E1 12.1eV 32 (b) The photon returns to the ground state either directly by emitting one photon of energy 12.1 eV, or return first to the n = 2 state by emitting a photon of energy E3 E2 1.5 (3.4) 1.9eV and then from the n = 2 state to the ground state by emitting a photon of energy E2 E1 3.4 (13.6) 10.2eV 23. A hydrogen atom is in its ground state ( n 1). Using the Bohr theory of the atom, calculate (a) the radius of the orbit, (b) the linear momentum of the electron, (c) the angular momentum of the electron, (d) the kinetic energy, (e) the potential energy, and (f) the total energy. (a) rn n 2 a0 r1 a0 0.529 A (b) pc 2mc 2 E 2 0.511 10 6 13.6 3728eV p 2 10 26 kg.m / s (c) L 6.582 10 16 eV .s (d) KE E 13.6eV (e) PE 27.2eV (d) E 13.6eV 26. Calculate the longest and shortest wavelengths in the Lyman series for hydrogen, indicating the underlying electronic transition that gives rise to each. Are any of the Lyman spectral lines in the visible spectrum? Explain. 1 1 R 2 2 , n 2,3,4,..., R 1.097 10 7 m 1 n 1 1 1 Longest 4 1 121nm , shortest 91nm . None of the Lyman lines is in the visible 3R R spectrum. 37. Use Bohr’s model of the hydrogen atom to show that when an atom makes a transition from the state n to the state n 1 , the frequency of the emitted light is given by f 2 2 me k 2 e 4 2n 1 2 2 h3 n 1 n Show that as n , the expression above varies as 1 n3 and reduces to the classical frequency one would expect the atom to emit. (Hint: To calculate the classical frequency, note that the frequency of revolution is v n2 2 , where r is given by rn ). This is an 2r me ke2 example of the correspondence principle which requires that the classical and quantum models agree for large values of n . mvr n ke2 mv 2 n2 2 rn r r2 mke2 ke2 ke2 mke2 k 2 me 1 1 2 ke2 En 2 2 KE mv v 2rn 2 2 n n 2 2 n 2 ke2 v mk 2 e 4 4 2 mk 2 e 4 1 n f 3 3 3 3 2r 2 n 2 2 2 n h n mke2 We also have E hf En En1 k 2 me 4 2 2 1 1 2 2 mk 2 e 4 f n 12 n 2 h3 2 2n 1 4 2 mk 2 e 4 1 3 3 n 12 n 2 n h n