Bohr`s Atomic Model
advertisement
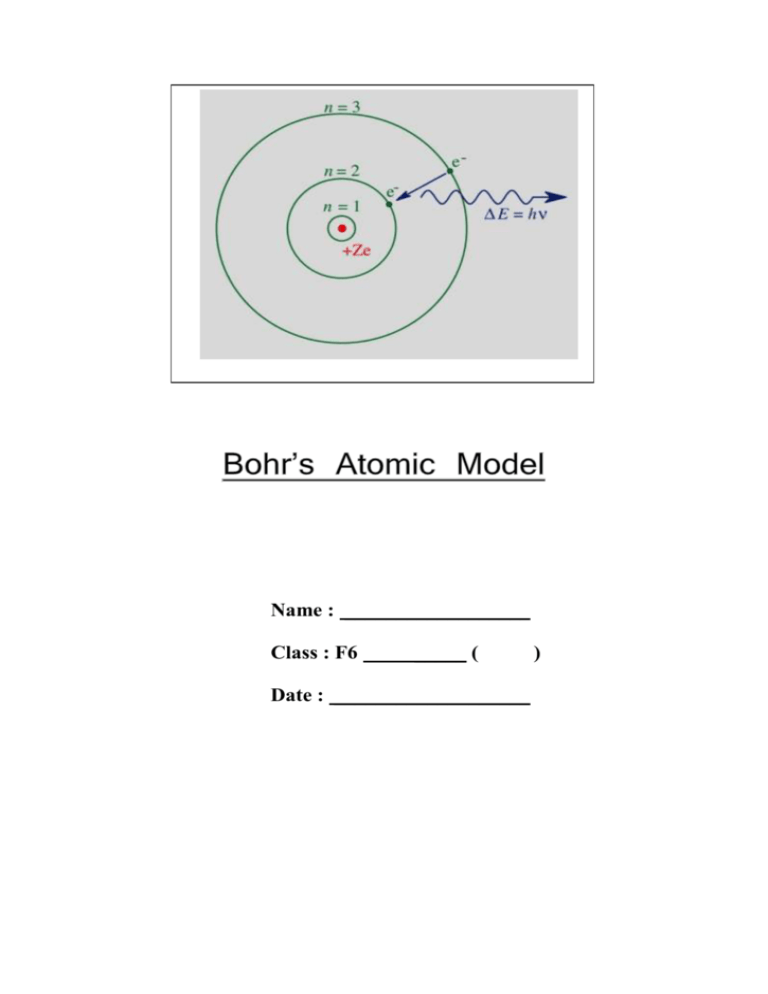
Name : __________________ Class : F6 _____ ( Date : ___________________ ) Bohr’s Atomic Model Multiple Choice Questions 1 Which of the following is/are the unit(s) of the Planck constant? (1) eV (2) kg m s−1 (3) J s A. (1) only B. (3) only C. (1) and (2) only D. (2) and (3) only Answer: B 2 Which of the following objects will emit a line spectrum? (1) a red-hot charcoal (2) an operating low pressure sodium lamp (3) an operating filament bulb A. (1) only B. (2) only C. (1) and (3) only D. (2) and (3) only Answer: B 3 When a hydrogen atom transits from the fourth excited state to the first excited state, a photon is emitted. Find the wavelength of the photon. A. 95.2 nm B 435 nm C. 488 nm D. 504 nm Answer: B 4 Which of the following is/are a postulate(s) of the Bohr model? (1) Electrons do not emit radiation when they are in the stationary states. (2) There is no electric force acting on an electron when it is orbiting round the hydrogen nucleus in a stationary orbit. (3) An atom will absorb an incident photon when the frequency of the photon is above a certain threshold value. A. (1) only B. (1) and (2) only C. (2) and (3) only D. (1), (2) and (3) Answer: A 5 A hydrogen atom is initially in the ground state. After absorbing a photon it is excited to the n = 6 state. Find the frequency of the incident photon. A. 9.12 × 1013 Hz B. 2.35 × 1014 Hz C. 3.19 × 1015 Hz D. 3.22 × 1015 Hz Answer: C 6 Which of the following best shows the energy level diagram of a hydrogen atom? Note that the vertical axis is drawn to a linear scale. A. B. C. D. Answer: D 7 Which of the following is not a possible energy level for a hydrogen atom? A. −6.04 eV B. −1.51 eV C. −0.544 eV D. −0.136 eV Answer: A 8 Which of the following is not a possible value for the angular momentum of an electron in a hydrogen atom? A. 5.28 × 10−35 J s B. 1.06 × 10−34 J s C. 5.28 × 10−34 J s D. 6.33 × 10−34 J s Answer: A 9 A hydrogen atom is in the second excited state. Find the minimum energy needed to ionize this atom. A. 0.850 eV B. 1.51 eV C. 3.40 eV D. 13.6 eV Answer: B 10 Which of the following statements about the ionization energy of an atom is/are correct? (1) It is the minimum energy required to remove an electron from an atom. (2) It is the energy required to excite an atom from an orbit of lower energy level to an orbit of higher energy level. (3) It must be equal to the negative of the ground state energy of the atom. A. (1) only B. (1) and (2) only C. (2) and (3) only D. (1), (2) and (3) Answer: A 11 The Pfund series of the hydrogen emission spectrum contains emission lines from transitions ending on the n = 5 level. Find the emission line with the longest wavelength in the Pfund series. A. 2290 nm B. 6120 nm C. 7480 nm D. 9210 nm Answer: C 12 In the following energy level diagram, the vertical axis is drawn to a linear scale. Which transition will give an emission of photon of the shortest wavelength? A. B. C. D. P Q R S Answer: A 13 Which of the following statements about the spectral lines of the hydrogen atom is/are correct? (1) The line with the longest wavelength in the hydrogen emission spectrum is resulted from the transition between the n = ∞ level and the n = 1 level. (2) Each spectral line is resulted from an atomic transition in which only one photon is emitted or absorbed. (3) Absorption spectrum is obtained when a sample of low pressure hydrogen gas is bombarded by a beam of energetic electrons. A. (1) only B. (2) only C. (1) and (3) only D. (2) and (3) only Answer: B 14 Which of the following figures correctly shows the diagram representing the transitions of the Lyman series in the hydrogen absorption spectrum? A. B. C. D. Answer: D 15 A sample of low pressure hydrogen gas is bombarded by a beam of electrons as shown. There is a loss of kinetic energy in the emergent electrons. Assume the change in kinetic energy of the gas molecules is negligible. Which of the following statement is/are incorrect? (1) The collisions between the gas molecules and the electrons are elastic. (2) Photons will be emitted by the sample in random directions. (3) A. B. C. D. The sample will emit a continuous spectrum. (2) only (3) only (1) and (2) only (1) and (3) only Answer: D Structured Questions 1 (a) State the postulates of the Bohr model. (4 marks) (b) State a piece of experimental evidence that supports the existence of energy levels in an atom. (1 mark) (c) Suggest two ways to excite a sample of hydrogen atoms. (2 marks) Answer: (a) 1. Electrons move in circular orbits around the nucleus and classical laws of circular motion and electric forces are valid. (1A) 2. The electron can only move in stationary orbits in which it does not radiate while accelerating. (1A) 3. The angular momentum of the electron in a stationary orbit is quantized that h can only be integral multiples of . (1A) 2π 4. The atom emits or absorbs radiation in the form of a photon when it transits from one energy level to another. (1A) (b) A heated low pressure gas emits discrete spectral lines of specific wavelengths. (c) 1. Bombard the sample of atoms with energetic particles. (1A) 2. Illuminate the sample with a continuous spectrum of electromagnetic radiation such as white light. (1A) 2 The following figure shows the lowest five energy levels of a hydrogen atom. Initially, the atom is in the first excited state. (a) If the atom absorbs a photon of energy 4.08 × 10−19 J. Sketch in the above figure an arrow representing the transition. (3 marks) (b) The excited atom emits a photon and transits to the ground state afterwards. (i) Sketch in the above figure another arrow representing this transition. (1 mark) (ii) Find the wavelength of the emitted photon. (2 marks) Answer: (a) The energy of the photon 4.08 10 19 2.55 eV . 1.60 10 19 (1M) ( Since 3.40 0.850 2.55 eV , the photon can excite the atom from the first excited state (−3.40 eV) to the third excited state (−0.850 eV). (1M) The transition of the atom is shown below. (1A) (b) (i) The transition of the atom is shown below. (1A) (ii) Applying E hc , the wavelength of the emitted photon is 6.63 10 34 3 108 9.75 10 8 m hc E 13.6 0.850 1.60 10 19 (1M+1A)








