Hydrogen Atom
advertisement
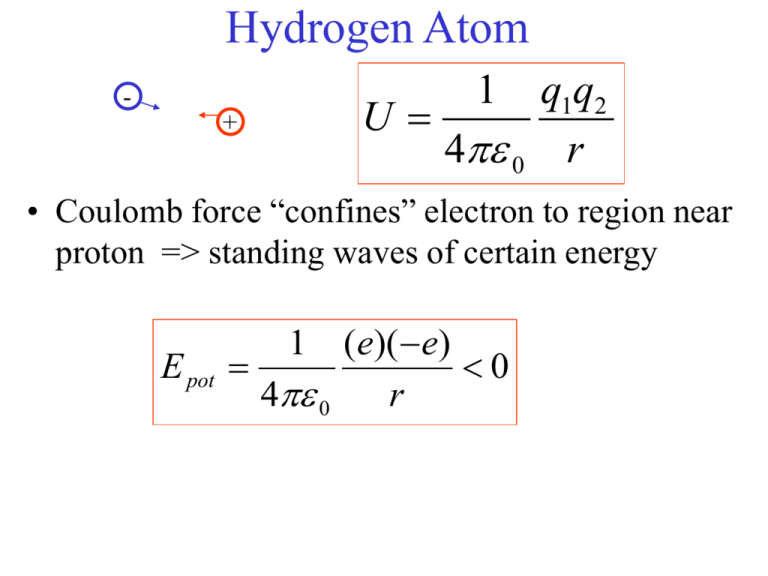
Hydrogen Atom 1 q1q2 U 4 0 r + • Coulomb force “confines” electron to region near proton => standing waves of certain energy E pot (e)( e) 0 4 0 r 1 Electron in n=2 level makes a transition to lower level by emitting a photon of frequency f=E/h = (E2-E1)/h =c/ Transitions • Electron in ground state E1 can move to the excited state E3 if it absorbs energy equal to E3-E1 • absorb a photon hf= E3-E1 • electron will not stay in the excited state for long => emits a photon or a sequence of photons 3 hf= E3 - E1 2 hf = E3 - E2 = hc/ 1 hf = E2 - E1 = hc/ Emission spectrum photon Continuous visible spectrum Line spectra from H, He, Ba, Hg Hydrogen Atom + q1q2 U k r • Coulomb force “confines” electron to region near proton => standing waves of certain energy (e)( e) U k 0 r Atoms • In 1913 Neils Bohr proposed a model of hydrogen based on a particle in an orbit • electron with charge -e in a circular orbit about a nucleus of charge +Ze • Coulomb attraction provides centripetal force mv2/r= kZe2/r2 • potential energy is U= kq1q2/r = -kZe2/r • kinetic energy K=(1/2)mv2=(1/2)kZe2/r • hence U= -2K (same for gravity!) • total E= K +U = -K = -(1/2)kZe2/r • e/m theory states that an accelerating charge radiates energy! • Should spiral into the nucleus! • Why are atoms stable? Bohr’s postulates • Bohr postulated that only certain orbits were stable and that an atom only radiated energy when it made a transition from one level to another • the energy of a photon emitted was hf = Ei - Ef • since the energies of the orbits are related to their radii, f= (1/2)(kZe2/h)(1/r2 - 1/r1) • experimentally observed photon frequencies satisfied f=c/ = cR(1/n22 - 1/n12) where n1 and n2 are integers Rydberg-Ritz formula • do the allowed values of r n2 ? • If we think of the allowed orbits as standing waves then we need 2r= n for constructive interference Stable orbits • • • • • 2r= n for constructive interference but de Broglie says =h/p hence pr= nh/2 but L=rp for circular orbits hence L= mvr =n ħ n=1,2,3,… angular momentum is quantized! Bohr Atom Bohr Theory • • • • How do we find the allowed radii? Coulomb force = kZe2/r2 = mv2/r => v2=kZe2/mr but Bohr says mvr= n ħ => v2= n2 ħ2/m2r2 solve for r: r= n2 (ħ2/mkZe2) = n2 a0/Z where a0 is a radius corresponding to n=1 and Z=1(Hydrogen) • a0 = ħ2/mke2 = 0.0529 nm (called the Bohr radius) • hence only certain orbits are allowed => only certain energies • energy differences = (1/2)kZe2(1/r2 - 1/r1) = (1/2)(kZ2e2/a0)(1/n22 - 1/n12) Bohr Atom • compare with Rydberg-Ritz formula for observed wavelengths in Hydrogen 1/ = R(1/n22 - 1/n12) where R is Rydberg constant • frequency of photons f=c/= c R(1/n22 - 1/n12)= (E2 - E1)/h • using Z=1, R=mk2e4/4cħ3 =1.096776 x 107 m-1 in agreement with experiment! • Energy levels can be determined from allowed radii • E=-(1/2)kZe2/r = -(mk2e4/2ħ2)(Z2/n2) = -E0 Z2/n2 • E0 is the lowest energy for hydrogen = 13.6 eV • hence hydrogen atom(Z=1) has energies En = -13.6eV/n2 n=1,2,... (e)( e) U k 0 r E1 = -13.6eV En = -13.6eV/n2 n=1,2,3,... Hydrogen Atom • En = -13.6eV/n2 n=1,2,3,… • ground state has E1 = -13.6eV • ionization energy is 0- E1 = 13.6eV => energy needed to remove electron • excited state: n=2 E2 = -(13.6/4)eV • electron must absorb a photon of energy hf= E2 - E1 =hc/ = (3/4)(13.6eV) Electron in n=2 level makes a transition to lower level by emitting a photon of frequency f=E/h = (E2-E1)/h =c/ En-E2 = 13.6eV ( 1/4 -1/n2) = hf = hc/ max=hc/(13.6eV)(5/36) ~ 658 nm => 4 lines visible En-E1 = 13.6eV ( 1 -1/n2) = hf = hc/ max=hc/(13.6eV)(.75) ~ 122 nm Balmer Series











