4 The Particle Nature of Matter
advertisement
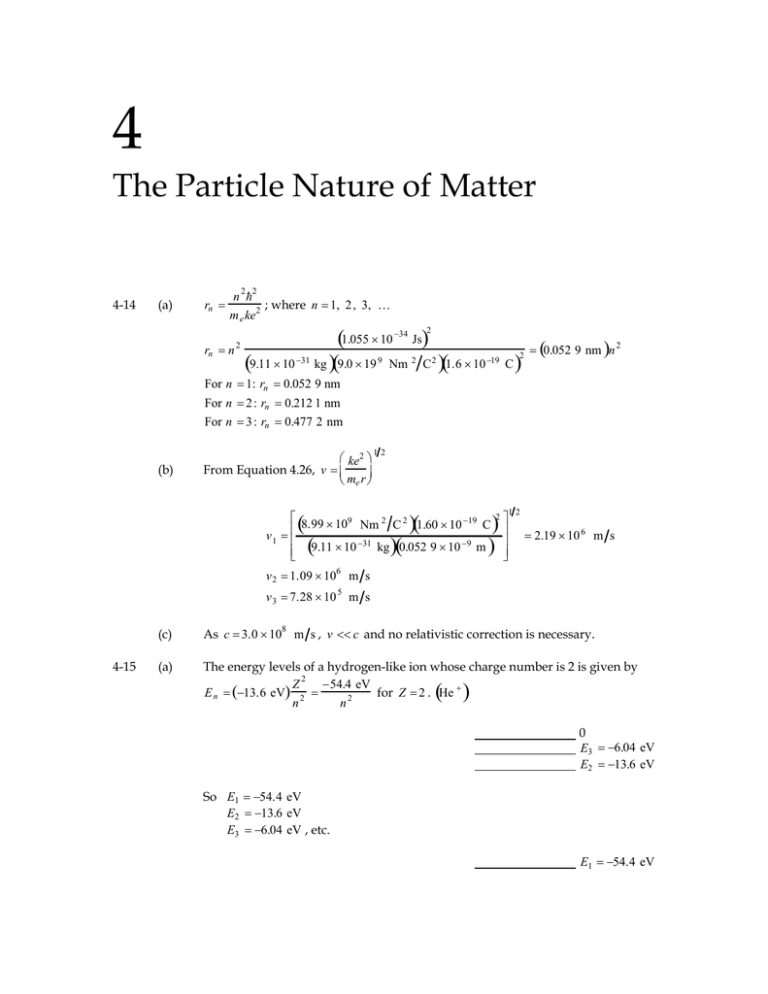
4 The Particle Nature of Matter 4-14 (a) n 2 2 rn 2 ; where n 1, 2 , 3, m e ke rn n 2 1.055 10 34 Js 2 2 0.052 9 nm n 31 9 2 2 19 9.11 10 kg 9.0 19 Nm C 1.6 10 C 2 For n 1: rn 0.052 9 nm For n 2: rn 0.212 1 nm For n 3: rn 0.477 2 nm 12 (b) ke2 From Equation 4.26, v me r 2 8.99 109 Nm 2 C 2 1.60 10 19 C v 1 9.11 10 31 kg 0.052 9 10 9 m 12 2.19 10 6 m s v 2 1.09 106 m s v 3 7.28 10 4-15 5 m s (c) As c 3.0 108 m s , v c and no relativistic correction is necessary. (a) The energy levels of a hydrogen-like ion whose charge number is 2 is given by 2 54.4 eV Z E n 13.6 eV 2 for Z 2 . He 2 n n 0 E3 6.04 eV E2 13.6 eV So E1 54.4 eV E2 13.6 eV E3 6.04 eV , etc. E1 54.4 eV (b) For He , Z 2 so we see that the ionization energy (the energy required to take the 2 54.4 eV 2 electron from the state n 1 to the state n is E 13.6 eV 2 for 2 1 n Z 2 . He 4-16 For Li 2 , Z 3 from Equation 4.36 0 E3 13.6 eV En 13.6Z 2 2 n eV 122.4 2 n eV E2 30.6 eV So E1 122.4 eV E2 30.6 eV E3 13.6 eV , etc. E1 122.4 eV 4-17 n 2 2 n 2 2 r Z 2 2 ; n 1 Zm ke m ke e e 2 34 1.055 10 Js 1 r Z 9.11 10 31 kg 9 10 9 Nm 2 C2 1.6 10 19 C 4-18 2 11 m 5.30 10 Z (a) For He , Z 2 , r 5.30 10 11 m 2.65 10 11 m 0.026 5 nm 2 (b) For Li 2 , Z 3 , r 5.30 10 11 m 1.77 10 11 m 0.017 7 nm 3 (c) For Be3 , Z 4 , r 5.30 10 11 m 1.32 10 11 m 0.013 2 nm 4 (a) 1 1 E 13.6 eV 2 2 12.1 eV 1 3 (b) 1 1 Either E 12.1 eV or E 13.6 eV 2 10.2 eV and 1 2 1 1 E 13.6 eV 2 2 1.89 eV . 2 3 4-21 (a) For the Paschen series; 1 1 R 2 2 ; max 1 874.606 nm . For minimum wavelength, n i , 3 4 9 1 1 R 2 ; min 820.140 nm . 3 R 1 ni 4 , 1 min (b) hc min hc min 4-22 E K U 1 1 R 2 2 ; the maximum wavelength corresponds to 3 n i 1 max 1.6 10 J eV hc 1 874.606 nm 19 hc 820.140 nm 1.6 10 19 J eV 0.662 7 nm 1.515 nm 2 mv 2 1 ke2 mv 2 ke2 1 ke U . But . Thus E , so 2 r 2 r 2 2 r 2 U 2E 2 13.6 eV 27.2 eV and K E U 13.6 eV 27.2 eV 13.6 eV . 4-23 (a) r1 0.052 9 nm n 0.052 9 nm (when n 1 ) (b) ke2 m e v m e me r 2 12 9.1 10 31 kg 9 109 Nm 2 C 2 m e 5.29 10 11 m M e v 1.99 10 24 kg m s 4-25 12 1.6 10 19 C (c) L m e vr 1.99 10 24 kg m s 5.29 10 11 m , L 1.05 10 34 kg m 2 s (d) K E 13.6 eV (e) U 2K 27.2 eV (f) E K U 13.6 eV (a) 1 1 16 1 1 9 14 E hf 13.6 eV 2 2 or f 13.6 eV 1.60 10 Hz 15 4.14 10 eV s n f n i (b) T 2 rn v 12 so frev r3 3 a0 and 2 12 ke2 ke2 1 v . Using v , frev . For n 3 , T 2 rn me rn mrn frev 8.99 10 9 Nm 9.11 10 2 C 2 1.60 10 19 C 2 3.149 5.29 10 11 m 31 kg 9 5.29 10 11 m 2 12 frev 2.44 1014 Hz n 3 frev 1.03 1014 Hz n 4 Thus the photon frequency is about halfway between the two frequencies of the revolution. 4-32 (a) 1H 1 9.109 390 10 31 kg 1.672 63 10 27 kg meM me M 9.109 390 10 31 kg 1.672 63 10 27 kg 9.109 390 10 31 kg 1.672 63 10 27 kg 9.104 431 10 31 kg 0.000 910 939 0 10 27 kg 1.672 63 10 27 kg me 1 k n f 2 31 kg 1 9.104 431 6 10 1 7 1 1 2 1.097 315 3 10 m 31 2 2 2 n i 9.109 390 10 3 kg 1H 656.469 1 nm 4-33 (b) Similarly, we find 2H 656.292 5 nm . (c) 3H 656.232 5 nm 2 1.05 10 34 Js n 2 a0 n 2 2 2 rn 1 3.1 10 15 m . Note 2 Z 9 2 19 mke2 Z 207 m e 8.99 10 Nm C 1.60 10 C 82 that this means the muon grazes the nuclear surface, and so experiments with muonic atoms give information about the nuclear charge distribution. ke Z 2a n ke Z 2 En 2 2 2 0 2 2 2 n mke 2 2 2 4 mk e Z 2 2 2 n 2 Using m 207me , n 1 , and Z 82 yields E1 18.9 MeV . (Using the reduced mass makes no difference in the answers to three significant figures.) 4-35 2 2 meM me me n since m e M . In general, rn 2 , so for positronium Z 1 , me M 2 2 Z ke 2 n 2 m e 2 and rpositronium ke 2a0 n 2 2rhydrogen . Similarly, 1 2 E hydrogen 6.80 eV . Epositronium 2 2 n 4-37 hf E 4 2 me k 2 e 4 2 h2 1 2 2 m e k 2 e 4 2n 1 1 f , 2 2 n 12 n 2 h3 n 1 n as n , 1 2 2 2 m e k 2 e 4 2 1 ke2 1 v . The revolution frequency is f f 3 3 2 where 3 2 r 2 h m e r n 12 1 ke2 r 2 substituting for r, f 2 me 4 m e ke2 n 2 h2 8 3 m ke3 m k 1 2 4 2 m k 2 e 4 e e e . n 3 h3 h3 n 3











