Electron Configuration and Quantum Numbers Worksheet #13
advertisement

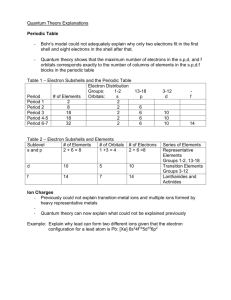
Electron Configuration and Quantum Numbers Worksheet #13 Do your work on notebook paper. 1. Give the name and symbol for each of the following elements: a. 1s22s22p63s1 b. 1s22s22p3 c. 1s22s22p63s23p63d34s2 d. 1s22s22p63s23p64s1 2. What is the maximum number of electrons in each of the following subshells? a) 2s b) 4p c) 3d d) 1p e) 4f f) 2p g) 2d h) 6g 3. What is the number of orbitals in each of the following subshells? a) 2s b) 4p c) 3d d) 1p e) 4f f) 2p g) 2d h) 6g 4. Write the electron configurations for the following elements. a) P b) Y c) Cs d) Re e) Cm f) He g) Ni 5. Draw the electron box diagrams (orbital notation – the arrows) for each of the following. a) P b) Y c) Cs d) Re e) Cm f) He g) Ni 6. Write the electron configuration for each of the following. a) P3b) Mg2+ c) Cs+ d) O2e) U3+ 7. What is the maximum number of electrons that can be associated with the following set of quantum numbers? a) (2,1, , ) b) (5, , , ) c) (4, 4, , ) d) (3, 2, -1, -½) e) (3, 0, 1, ) f) (4, 3, , ) g) (3, 3, -3, ) h) (3, , 1, ) 8. Given the following electron box diagram, write the set of quantum numbers for each electron that is marked. ↑ ↓ ______ 1s ↑↓ _______ 2s ↑ ↓ ↑↓ ↑ ↓ _______ _______ _______ 2p 2p 2p ↑ ↓ ______ 3s ↑ ↓ ↑ ↑ ______ ______ ______ 3p 3p 3p Circled = __________________ Boxed = _________________________ Diamond = _________________ Last one placed = __________________ 9. What element is the electron box diagram in question eight showing? 10. Draw the electron box diagram for chlorine. YOU MUST LABEL THE ORBITALS AND IT IS POSSIBLE THAT NOT EVERY BOX WILL BE USED. 11. What is the quantum numbers of the last electron placed in question 10?