Ch. 6 Outline & Objectives
advertisement

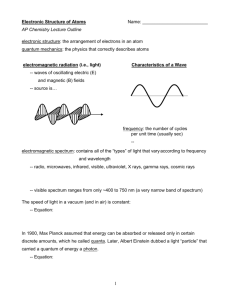
Honors Chemistry Mrs. Klingaman Chapter 6: Electron Structure of Atoms Objectives: the student will be able to: Understand the wave nature of light. Describe the electromagnetic spectrum. Perform calculations involving wavelength, frequency, energy and the speed of light. Perform calculations involving the DeBroglie equation. Interpret basic line spectra for selected gases. Define the uncertainty principle. Assign electron configurations to atoms and ions. Assign quantum numbers to electrons. Identify core and valence electrons using the periodic table. Define and apply orbital filling rules. Draw orbital diagrams. Problem Sets: Suggested problems you can do and check the answers in the back of the textbook. Pg. 253: 13, 15, 17, (radiant energy) Pg. 253: 19, 23, 25 (quantized energy) Pg. 254: 33, 37, 39, 40, 41 (Bohr’s Model) Pg. 254: 47, 49, 51, 53 (quantum mechanics & atomic orbitals) Pg. 255: 63, 66, 67, 71 (electron configuration) Honors Chemistry Reading Guide Chapter 6: Electron Structure of Atoms 6.1 - The Wave Nature of Light 1. Define electronmagnetic radiation and list its properties. 2. Define properties and “parts” of a wave (diagrams are nice ). 3. What is the speed of light? Include a formula, numerical value, units and relationship of wavelength and frequency in your definition. 4. Review metric prefixes identified in Table 6.1 and relate them to length and type of radiation. 6.2 - Quantized Energy and Photons 1. What is the relationship between hot objects and quantized energy? 2. Explain the relationship between quantum of energy and Planck’s constant. Be sure to include the equation for energy in your discussion. 3. Explain the photoelectric effect and role of photons. 4. Why is light (electromagnetic radiation) described as having both wavelike and particle-like characteristics? 6.3 - Line Spectra and the Bohr model 1. Explain the concept of radiation as it relates to wavelength, energy and spectrum (continuous vs. line spectrum) 2. Identify the three postulates that are the basis of Bohr’s model of electron orbitals. 3. What is the principal quantum number (n)? 4. Explain the relationship between ground state and excited state electrons. 5. What were the most significant contributions of Bohr’s model to our current understanding of electron structure? 6.4 - The Wave Behavior of Matter 1. What is meant by the term “matter waves”, especially as it applies to electrons and what is the equation to describe this property? 2. Explain the Heisenberg Uncertainty principle as it applies to electrons (no equation required). 6.5 - Quantum Mechanics and Atomic Orbitals 1. Define electron density. 2. What is the principle quantum number (n)? 3. What is the second quantum number (azimuthal, l)? 4. What is the magnetic quantum number (m)? 6.6 - Representation of Orbitals 1. Describe properties of the s-orbital. 2. Describe properties of the p-orbital. 3. Describe properties of the d and f orbitals. 6.7 - Many-electron Atoms 1. What is electron spin and how does this relate to the spin magnetic quantum number? 2. What is the Pauli exclusion principle? 6.8 - Electron Configurations 1. What is electron configuration? 2. Define orbital filling rules. 3. What is meant by “paired electrons” and “unpaired electron”? 4. What is Hunds Rule? 5. Given an example and explain the condensed (noble gas) configuration. 6. Explain the filling order for transition metals (d-block elements). 7. Explain the filling order for lanthanides and actinides (f-block elements). 6.9 - Electron Configurations and the Periodic Table 8. Define what is meant by representative or main-group (core) elements. 9. What are valence electrons? 10. Identify those elements which defy normal filling rules and write their electron configuration.