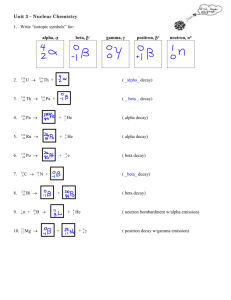
WS nuclear decay
advertisement
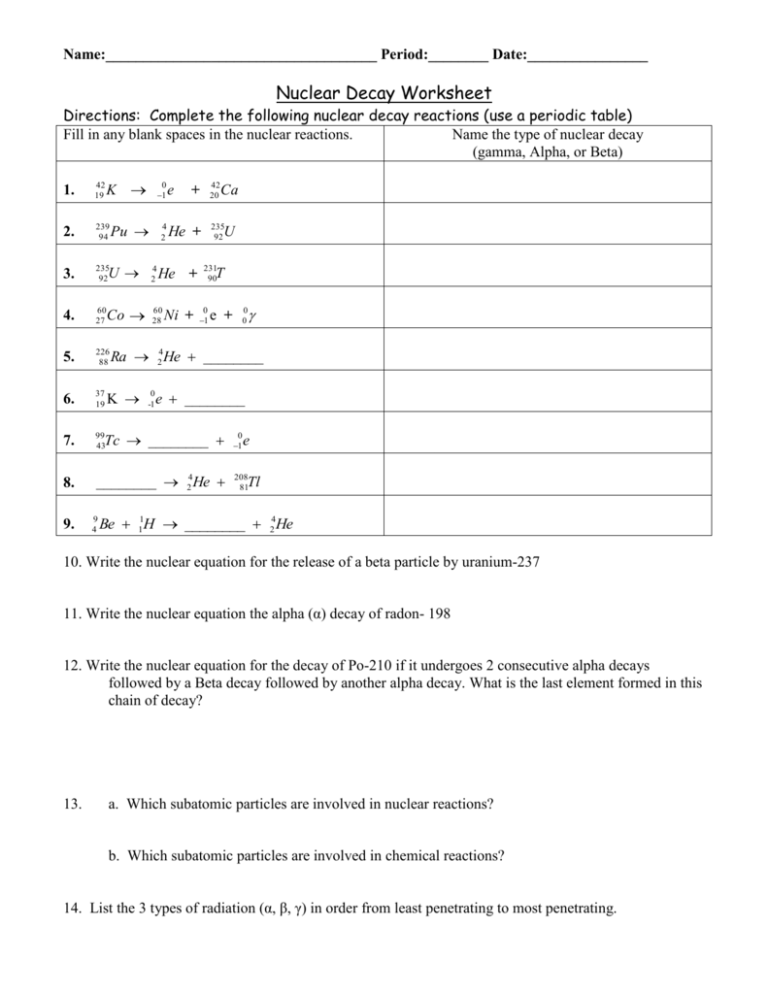
Name:____________________________________ Period:________ Date:________________ Nuclear Decay Worksheet Directions: Complete the following nuclear decay reactions (use a periodic table) Fill in any blank spaces in the nuclear reactions. Name the type of nuclear decay (gamma, Alpha, or Beta) K 0 1 Pu 4 2 1. 42 19 2. 239 94 3. 235 92 4. 60 27 5. 226 88 6. 37 19 7. 99 43 0 1 8. ________ 24He 208 81 9. U 4 2 Co 60 28 e 42 20 + 235 92 U He + He + Ni + Ca 231 90 T 0 1 Ra 24He ________ K 0 -1 e ________ Tc ________ 9 4 0 0 e + e Tl Be 11H ________ 24He 10. Write the nuclear equation for the release of a beta particle by uranium-237 11. Write the nuclear equation the alpha (α) decay of radon- 198 12. Write the nuclear equation for the decay of Po-210 if it undergoes 2 consecutive alpha decays followed by a Beta decay followed by another alpha decay. What is the last element formed in this chain of decay? 13. a. Which subatomic particles are involved in nuclear reactions? b. Which subatomic particles are involved in chemical reactions? 14. List the 3 types of radiation (α, β, γ) in order from least penetrating to most penetrating. 15. Fill blank boxes in the table below to summarize the three types of nuclear decay Property Composition Alpha radiation Beta radiation Gamma radiation Alpha particle (He nucleus) Symbol , 0 1 e Penetrating power Very high Effective Materials to Shield/Protect from radiation 16. Half-life Problems: a) an isotope of cesium-137 has a half-life of 30 years. If 1 gram of cescium-137 disintegrates over a period of 90 years, how many grams of cesium-137 would remain? b) 384 grams of chromium-48 decays. After 6 half-lives, how many grams of the original nuclei would remain? c) If we start with 400 atoms of a radioactive substance, how many atoms would remain after one halflife? ________________ how many are left after two half-lives _______________? How many are left after three half-lives? _________________________ how many left after four half-lives?____________ d) Based on the graph: -How many years is one half-life for carbon-14?________________ -How many years will it be for carbon-14 to have three halflives?________________ -If only 25% of the original radioactive material is left, how many years have passed? ___________________ 16. Thorium-238 undergoes radioactive decay until a stable isotope is reached. Write the reactions for the decay of Th-238. There are eleven steps beginning with Alpha decay with each product becoming the reactant of the next decay. Circle the final Stable isotope. • Alpha: • Beta: • Beta: • Alpha: • Alpha: • Alpha: • Alpha: • Beta: • Beta: • Alpha: • Beta: Extra questions: DO NOT PRINT Explain how energy loss and nuclear stability are related to radioactive decay. What is the primary factor determining whether or not an atom is stable or unstable? What is mass defect and why is it important? What is the difference between nuclear fusion and nuclear fission?