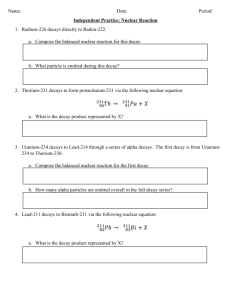
Balancing nuclear equations
advertisement

Balancing nuclear equations 1. What radiation will be emitted when Bi-211 decomposes to Tl-201? In this problem, the reactant is Bi-211 and the products are Tl-201 and unknown radiation. 211 Bi 83 → 20781Tl + ? (i) Check the mass numbers (or superscripts): 211 → 207 + x (ii) Check the atomic numbers (subscripts): 83 → 81 + y Therefore: x = 4 and y = 2. Which particle has a mass of 4 and a charge of +2? Answer: alpha particles The balanced nuclear equation is: 211 83Bi → 20781Tl + 42He. The balanced nuclear equation shows alpha particle as one of the products. This means that the nuclear decay involves the loss of an alpha particle from a nucleus. Scientists call this reaction an alpha decay. 2. Which of these equations is balanced? 226 Ra 88 → 22286Rn + 42He + γ 226 Ra 88 → 22286Rn + 42 He Answer: Both are balanced because in each equation the sum of superscripts of the left equal to that on the right, and the sum of subscripts on the left equal to that on the right. This is an alpha decay process. Example sheet