Spectrum of Hydrogen
advertisement

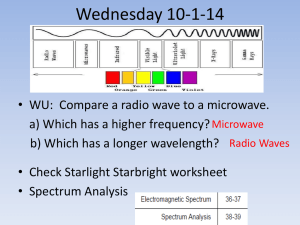
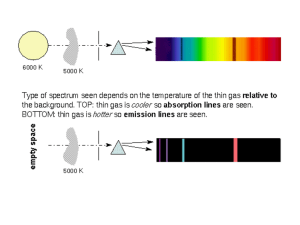
The Visible Atomic Spectrum Of Hydrogen Many substances give off visible light when energetically excited in an electric discharge tube or when heated. The light is emitted only at certain wavelengths characteristic of the substance. When this light is passed through a prism or reflected off a diffraction grating, the various wavelengths are dispersed and each appears as a separate line in what is called the emission (line) spectrum. In this experiment you will observe and measure the line spectrum of hydrogen, and determine the energies of several of the lower electronic energy states of the hydrogen atom. The Electromagnetic Spectrum Electromagnetic radiation (light) consists of electric and magnetic fields oscillating at right angles to each other and traveling through space. The oscillating fields are waves, characterized by a wavelength, , and a frequency, . For any wave motion, the product of wavelength and frequency is the speed of the wave. For electromagnetic radiation this is, = c [eqn. 1] where c is the speed of light in a vacuum (2.998 x 108 m/s)For electromagnetic radiation, common wavelength units are meters (m), centimeters (cm) and nanometers (nm). The frequency unit is cycles per second (s-1), also called hertz (Hz). The regions of the electromagnetic spectrum are shown in Figure 1. Our eyes are sensitive to a rather narrow band of wavelengths between roughly 400 nm (violet) and 700 nm (red). The part of the hydrogen spectrum involved in this experiment is in this visible region of the electromagnetic spectrum. In addition to wave characteristics, electromagnetic radiation also can be considered as particles, called photons. The energy, E, of a photon is proportional to its frequency, . Ephoton = h [eqn. 2] where h is Planck’s constant (h=6.626 x 10-34 J·s). When this value of h is multiplied by frequency, , in Hz (s-1), the energy is in joules (J). Combining eqn. 1 and 2 gives, Red Orange Yellow Green Blue [eqn. 3] Indigo hc/ Violet Ephoton = Visible rays 10-5 X-rays 10-3 10-1 Ultraviolet 10 Infra-red 103 Microwaves 105 107 Radio waves 109 1011 wavelength, (nm) Figure 1: The electromagnetic spectrum. Armando Herbelin 687319500 Page 1/7 2/6/2016 Atomic emission (line) spectra According to quantum mechanics, electrons can exist only in certain states called orbitals. Each orbital has a specified energy and "shape" which describes the probabilities of finding the electron in any region of space around the nucleus. When an electron in an atom changes its state it absorbs or emits energy equal to the difference between the energies of the initial (Ei) and final (Ef) states involved in the change. When atoms are energetically excited, energy is absorbed. The emission (line) spectrum results when excited atoms fall from higher (Ei) to lower (Ef) energy states emitting light. The energy of the emitted photon corresponds to the difference between the higher and lower state energies. E = Ei - Ef = Ephoton = h [eqn. 4] The emitted light is dispersed by a prism as shown in Figure 2. The Spectroscope A spectroscope is an instrument which separates light into its various wavelength components. The gas to be studied is in a gas discharge tube placed next to the slit on the spectroscope. When a sufficiently high potential difference (voltage) exists between the two electrodes in the tube, electrons flow, colliding with the gas atoms in the tube. These collisions excite atoms to higher energy levels. When they fall to lower energy levels, light is emitted. You will use a Bunsen spectroscope (Figure 3). The emitted light enters the spectroscope through a slit, is collimated by a lens and refracted into the various wavelengths by the prism. Each wavelength gives a vertical line in the spectrum. One arm of the spectroscope contains a linear scale which is illuminated with a small external light, superimposing the linear scale on the spectrum as viewed through the eyepiece. When calibrated this scale is used to determine the wavelengths of the hydrogen atom spectrum. Calibration of the spectroscope The first task is to construct a calibration curve which will enable you to convert the arbitrary scale readings of the spectroscope to corresponding wavelengths. The known wavelengths of the mercury (Hg) vapor spectrum will be used for this purpose. The instructor will show you how to place a mercury discharge tube in the power supply and turn it on. Set it as close as possible to the spectroscope slit. We only have one set of lamps (borrowed from the physics department) so be very careful. Before proceeding keep in mind the following hints on technique: Armando Herbelin 687319500 Page 2/7 2/6/2016 To avoid eyestrain, don't look in the eyepiece telescope for more than a minute. Look away occasionally. The brightness of the spectrum is very dependent on the position of the discharge tube in front of the slit. Move the discharge tube slightly while viewing the spectrum to find the best position. Rotate the eyepiece telescope so as to center the line being measured. The focus may change with wavelength; if so refocus the eyepiece. Open the slit to increase the intensity of a dim line; narrow the slit to sharpen bright lines. The scale reading for a line is somewhat dependent on the position of the viewer's head (this is called parallax). Reduce the parallax error by centering each line in the middle of the telescope. There are three adjustments on the spectroscope: 1. opening/closing the slit to adjust the intensity of the observed lines, 2. focusing the eyepiece, and 3. moving the telescope to center lines. Be sure you know what to turn to make these adjustments. When viewed in the spectroscope the mercury line spectrum is superimposed on an arbitrary linear scale. Since you want to calibrate this scale, you need to correctly identify the colored lines seen in the spectroscope with the wavelength values given in black-and-white sketch in Figure 4. We will construct a calibration curve is constructed from the wavelengths (nm) given in Figure 4 and the scale readings viewed in the spectroscope when all data have been collected. The moderately strong red line seen when the discharge tube is first turned on (cold) is the 691 run line; read this line first as it becomes very weak as the tube heats up. The bright yellow pair of lines (seen as a pair only at narrow slit width), is the 577/579 nm doublet. The lines at 546 nm and 436 nm are also reasonably intense. You should be able to identify the rest of the lines in Figure 4. Ignore very faint lines and the rainbow backgrounds. Record the scale reading, color, and wavelength for each of the eight mercury lines in a tabular format. Measuring the Atomic Spectrum of Hydrogen Place a hydrogen gas discharge tube in the power supply, set it next to the spectroscope slit and turn it on. Armando Herbelin 687319500 Page 3/7 2/6/2016 Adjust the slit opening until the lines are easily observed through the eyepiece. You should be able to see four lines although the line nearest the ultraviolet may be faint. Take scale readings for each observed line and record these scale readings in your notebook. Calculations 1. CALIBRATION OF THE SPECTROSCOPE In your notebook data table, add a column for the Log Spectroscope Reading. Compute these numbers using your calculator. Open the Excel template on your computer and select the Experiment worksheet. Fill in the table with your spectroscope readings and colors. Match the colors with the wavelengths from Figure 4. As you fill in the data, the plot will automatically update (NOTE: the plot shows Wavelength vs. Log(Spectroscope Reading) which should have your data in a line. If a point is off the line, you probably have the wrong wavelength). Record the slope, intercept, and R2 in your notebook. 2. ENERGY LEVELS OF HYDROGEN Although a complete description of atomic electron orbitals requires several quantum numbers, the orbital energy for hydrogen (and only hydrogen) is especially simple and is defined by a single quantum number. This is the principal quantum number, n, which can take only integer values (n = 1, 2, 3). The hydrogen energy levels are given by the expression, E 2 2 me2 n2h2 [eqn. 5] where m and e are the mass and charge of the electron, respectively. When values for e and m in Sl units are inserted, this equation gives the energy in joules (J). E 2.179 10 18 J n2 [eqn. 6] For n = 1, the electron is in the lowest (ground state) energy. From the above equation, the ground state energy is -2.179 x 10-18 J. The highest possible energy is zero (for n=). 18 Energy (J x 10 ) In the template, select the Specroscope Calibration worksheet. You will finish the table to calculate the hydrogen electron energy levels for n = 1 thru 6 and 1000. Enter a formula in cell B5 to calculate the energy (in Joules) based on equation 6. Hydrogen Energy Levels Be sure to use a reference to cell A5 for n. Copy the cell 0.0 and paste it over the rest of the table, cells B6 to B11. If you examine the formulas, you will find that the formulas -0.5 automatically adjust for row number (cell B6 uses n from A6 and so on). 3. ENERGY LEVEL DIAGRAM To get a visual picture of the meaning of these energy levels, construct a diagram similar to Figure 7.22 (pg 270) in your Chang text. The Excel graph is already configured for you but you need to scale the energies by 1018 so the graph label looks nice. The resulting energies will be attojoules. For the plot to look right, put the energy (in aJ) Armando Herbelin 687319500 Page 4/7 -1.0 -1.5 -2.0 -2.5 2/6/2016 in both columns D & E. We can use Excel’s constant function to do this. Enter the following formula into cell D5: =$B5*1E18 Now copy cell D5 and paste it over D5:E11. Note: The dollar sign before the B tells Excel not to modify the column letter when you paste the data across several rows. Print this spreadsheet (Use the Print Preview function to make sure it is all on one page). The levels on this diagram can be thought of as "rungs on an energy ladder." On a ladder you can only exist at heights corresponding to a rung, and when you change your position on the ladder, the change in height must equal to the difference between two rung heights. An electron can only exist on one of the allowed energy levels. When an electron changes levels, an amount of energy is emitted (or absorbed) equal to the difference between the two levels involved in the transition. 4. THE EMISSION (LINE) SPECTRUM OF HYDROGEN Use the calibration data (from section 1) to convert the scale reading for each hydrogen line to wavelength. To do this, enter the scale readings in the spreadsheet template, then calculate the log values. The Excel formula for log is: =log(B55). Finally, use y=mx+b with the slope and intercept to convert log scale reading into wavelength. The scientific version of this equation is: = m log(Scale Reading) + b. Recall that is the symbol for wavelength. Format the calculated wavelength as a number with one decimal places (round off to 0.1 nm). The four lines in the visible spectrum of hydrogen result from the excited state electrons with ni= 3, 4, 5, 6 and nf = 2. Note: ni is read is “n initial” – the electron’s initial energy level. They are part of the Balmer series of lines named after J.J. Balmer who in 1885 first showed that the wavelengths of the visible spectrum of hydrogen could be explained with a relatively simple formula. On the printout of the Energy Level Diagram for Hydrogen draw vertical arrows corresponding to the energy level transitions responsible for the four Balmer lines. If you want to keep the report electronic, open the View menu, Toolbars, drawing. There is an arrow tool that you can use to draw on the plot. Be sure not to move the graph after you draw arrows. When an electron in an excited level falls to a lower level, the energy of the emitted photon is obtained by combining equations 4, 5, and 6 to give equation 7. 1 1 E photon Ei E f 2.179 10 18 2 2 n i nf J or Armando Herbelin 687319500 Page 5/7 2/6/2016 1 1 E 2.179 10 18 2 2 n f ni [eqn. 7] J Equation 7 can be expressed in terms of the wavelength () of the emitted light. E photon hc 1 1 2.179 10 18 2 2 n n i f J [eqn. 8] hc E photon 1 1 2 2 n n i f (nm) 91.16 EXAMPLE: When an electron in a hydrogen atom falls from the n=2' level to the n= 1 level, the energy of the emitted photon is, 1 1 E photon 2.179 10 18 2 2 1 2 J 1.634 10 18 J The wavelength of the emitted light is, 1 1 121.5 nm 12 2 2 (nm) 91.16 This light is in the ultraviolet region of the electromagnetic spectrum and is not visible to our eyes. Theoretical wavelength values for the four hydrogen lines can be calculated using the concepts and formulas above. In the table of calculated wavelengths, enter values for nf and ni. Enter the nf =2 for all rows in your table. Enter 3, 4, 5, and 6 for ni. Calculate the four theoretical wavelengths using equation 8. Compare the theoretical wavelengths with your measured wavelengths. Comment on how well they match. Enter formulas to calculate the energies and frequencies associated with each of these lines. Use this table to answer questions 1 through 3. Questions (answer these in your notebook and then in the Excel template): 1. At what wavelength (nm) would you predict the n=5 to n=3 transition in hydrogen? Would the resulting line be in the visible, ultra-violet or infrared? Explain. 2. Calculate the energy of the photon emitted for the n=5 to n=3 transition. What is the frequency of the wave associated with this photon? 3. The amount of energy required to completely remove an electron from an atom is called the ionization energy. This corresponds to raising an electron to the highest possible energy state (n=). What is the ionization energy of a hydrogen atom in its ground state (n= 1)? 4. The energy value obtained in Question 2 has the units J/atom H. Convert this value to kJ/mol H and compare with the value given in chapter 8 of your text. Armando Herbelin 687319500 Page 6/7 2/6/2016 Name ___________________ Lab Section ___________________ Pre-Lab Exercises 1. How many lines do we expect to see for the hydrogen spectrum? Why? 2. The National Institute of Standards and Technology (NIST) identifies hydrogen lines at the following wavelengths (nm): 92.6226 389.0151 656.4665 93.7803 397.1195 954.859 97.2537 410.29 1005.22 102.5722 434.169 1094.11 121.5674 486.269 1282.16 383.6472 656.453 Which of these should we not be able to see? Why? 3. Use the Excel template (Prelab worksheet) to calculate the energy (J) and frequency (Hz) of each of these wavelengths. Do not spend time copying these results into your notebook – the electronic version will be sufficient. Armando Herbelin 687319500 Page 7/7 2/6/2016