blue 434 nm
advertisement
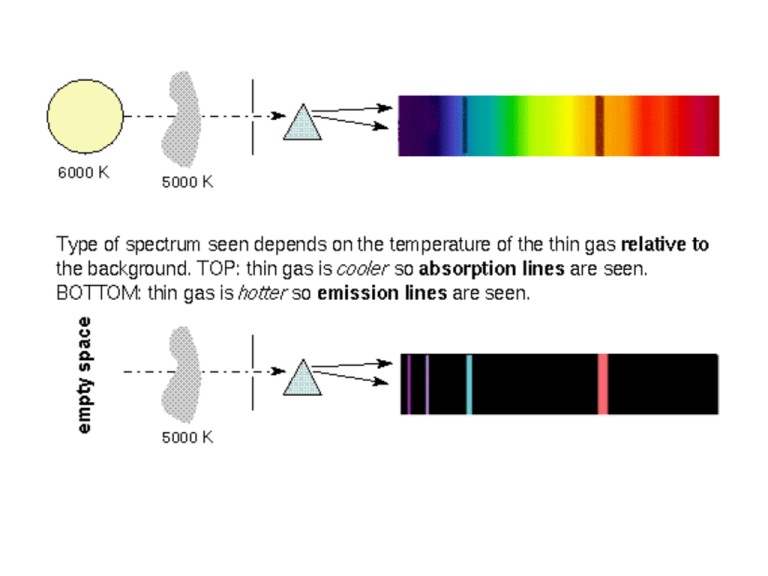
http://www.astronomynotes.com/light/discretc.gif Suppose two observers look at the spectrum of a cloud of gas in a laboratory; the first reports seeing emission lines and the second reports absorption lines. Explain. 1) Only the first observer sees the gas against a hot background 2) Only the second observer sees the gas against a hot background. 3) One observer is moving rapidly relative to the other. 4) The atoms in the gas are forming molecules. http://upload.wikimedia.org/wikipedia/commons/thumb/b/b2/Hydrogen_transitions.svg/2000px-Hydrogen_transitions.svg.png 656 nm 486 nm 434 nm 410 nm 656 nm: red-orange 486 nm: blue 434 nm: indigo 410 nm: violet http://www.daviddarling.info/images/hydrogen_spectrum.gif Atoms of different elements have unique spectral lines because each element 1) has atoms of a unique color. 2) has a unique set of neutrons. 3) has a unique set of electron orbits. 4) has unique photons. http://webmineral.com/help/FlameTest.shtml Atoms have particular associated spectral lines because 1) electrons have only certain allowed orbits. 2) light consists of waves. 3) light waves can show the Doppler effect. 4) speed of light in a vacuum is a constant. In the Sun, the transition from level 4 to level 2 of hydrogen produces photons with a wavelength of 486.1nm. In a star twice as hot as the Sun, this transition would produce photons with 1) half that wavelength. 2) the same wavelength. 3) twice that wavelength. 4) four times that wavelength. Here is the spectrum of a mystery star: Which elements are present? 1. 2. 3. 4. Calcium only Calcium and hydrogen Magnesium and calcium Calcium, hydrogen, and magnesium Here is the spectrum of a mystery star: Which elements are present? 1. 2. 3. 4. Calcium only Calcium and hydrogen Magnesium and hydrogen Calcium, hydrogen, and magnesium Decoding Cosmic Spectra http://www.pbs.org/wgbh/nova/space/decoding-cosmic-spectra.html Here is the spectrum of a mystery star: Which elements are present? 1. 2. 3. 4. Calcium, hydrogen, and magnesium Calcium and hydrogen Magnesium and hydrogen All of them http://abyss.uoregon.edu/~js/hc209/lectures/lec09.html http://jersey.uoregon.edu/prf/ THE SPECTRAL SEQUENCE Class O B A F G K M L T Spectrum ionized and neutral helium, weakened hydrogen neutral helium, stronger hydrogen strong hydrogen, ionized metals weaker hydrogen, ionized metals still weaker hydrogen, ionized and neutral metals weak hydrogen, neutral metals little or no hydrogen, neutral metals, molecules no hydrogen, metallic hydrides, alkalai metals methane bands Color Temperature bluish 31,500-49,000 K blue-white 10,000-31,500 K white 7500-10,000 K yellowish white 6000-7500 K yellowish 5300-6000 K orange 3800-5300 K reddish 2100-3800 K red-infrared 1200-2100 K infrared under 1200 K O B A F G K M L T Origins: Decoding Spectra of Light from Distant Galaxies Origins Hour 4, Back to the Beginning, Chapter 6 http://www.pbs.org/wgbh/nova/programs/ht/qt/3114_06.html











