Exam 4 chap 7 key
advertisement

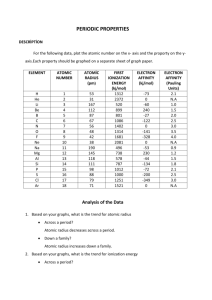
Exam 100 Points Name:____________________ Date: _______________ (3 points, 1 pt each)True or False: 1) Electron affinity measures how easy an atom gains an electron. Answer: TRUE 2) The atomic radius of iodine is one-half the distance separating the iodine nuclei. Answer: TRUE 3) A group of ions all containing the same number of electrons constitutes an isoelectronic series. Answer: TRUE (96 points, 4 pts each) Multiple Choice. Show all your work for partial credit 4) Of the following elements, which has the largest first ionization energy? A) B B) N C) P D) Si E) C Answer: B 5) In general, as you go across a period in the periodic table from left to right: (1) the atomic radius __________; (2) the electron affinity becomes __________ negative; and (3) the first ionization energy __________. A) decreases, decreasingly, increases B) increases, increasingly, decreases C) increases, increasingly, increases D) decreases, increasingly, increases E) decreases, increasingly, decreases Answer: D 6) Element M reacts with chlorine to form a compound with the formula MCl2 . Element M is more reactive than magnesium and has a smaller radius than barium. This element is __________. A) Sr B) K C) Na D) Ra E) Be Answer: A 7) Most of the elements on the periodic table are __________. A) gases B) nonmetals C) metalloids D) liquids E) metals Answer: E 8) The only noble gas that does not have the ns2 np6 valence electron configuration is __________. A) radon B) neon C) helium D) krypton E) All noble gases have the ns2 np6 valence electron configuration. Answer: C 9) Of the following, which gives the correct order for atomic radius for Mg, Na, P, Si and Ar? A) Mg > Na > P > Si > Ar B) Ar > Si > P > Na > Mg C) Si > P > Ar > Na > Mg D) Na > Mg > Si > P > Ar E) Ar > P > Si > Mg > Na Answer: D 10) Which one of the following atoms has the largest radius? A) O B) F C) S D) Cl E) Ne Answer: C 11) Which one of the following atoms has the largest radius? A) I B) Co C) Ba D) Sr E) Ca Answer: C 12) Which one of the following elements has the largest atomic radius? A) Se B) As C) S D) Sb E) Te Answer: D 13) Which one of the following elements has the largest atomic radius? A) O B) F C) Al D) P E) B Answer: C 14) Which of the following correctly lists the five atoms in order of increasing size (smallest to largest)? A) F < K < Ge < Br < Rb B) F < Ge < Br < K < Rb C) F < K < Br < Ge < Rb D) F < Br < Ge < K < Rb E) F < Br < Ge < Rb < K Answer: D 15) Of the choices below, which gives the order for first ionization energies? A) Cl > S > Al > Ar > Si B) Ar > Cl > S > Si > Al C) Al > Si > S > Cl > Ar D) Cl > S > Al > Si > Ar E) S > Si > Cl > Al > Ar Answer: B 16) Of the following atoms, which has the largest first ionization energy? A) Br B) O C) C D) P E) I Answer: B 17) Of the following elements, which has the largest first ionization energy? A) Na B) Al C) Se D) Cl E) Br Answer: D 18) Of the following elements, which has the largest first ionization energy? A) K B) Rb C) Sr D) Ca E) Ba Answer: D 19) Which equation correctly represents the first ionization of aluminum? A) Al- (g) Al (g) + eB) Al (g) Al- (g) + eC) Al (g) + e- Al- (g) D) Al (g) Al+ (g) + eE) Al+ (g) + e- Al (g) Answer: D 20) Which ion below has the largest radius? A) ClB) K+ C) Br D) FE) Na + Answer: C 21) The ion with the smallest diameter is __________. A) Br B) ClC) ID) FE) O 2Answer: D 22) Which of the following is an isoelectronic series? A) B5- , Si 4- , As3- , Te2B) F- , Cl- , Br - , IC) S, Cl, Ar, K D) Si 2- , P 2- , S2- , Cl2E) O2- , P- , Ne, Na + Answer: E 23) Which isoelectronic series is correctly arranged in order of increasing radius? A) K+ < Ca2+ < Ar < ClB) Cl- < Ar < K + < Ca 2+ C) Ca 2+ < Ar < K + < ClD) Ca 2+ < K + < Ar < ClE) Ca 2+ < K+ < Cl- < Ar Answer: D 24) Of the following elements, __________ has the most negative electron affinity. A) Na B) Li C) Be D) N E) F Answer: E 25) Chlorine is much more apt to exist as an anion than is sodium. This is because __________. A) chlorine is bigger than sodium B) chlorine has a greater ionization energy than sodium does C) chlorine has a greater electron affinity than sodium does D) chlorine is a gas and sodium is a solid E) chlorine is more metallic than sodium Answer: C 26) Of the elements below, __________ is the most metallic. A) Na B) Mg C) Al D) K E) Ar Answer: D 27) The list that correctly indicates the order of metallic character is __________. A) B > N > C B) F > Cl > S C) Si > P > S D) P > S > Se E) Na > K > Rb Answer: C 28) bonus point Pe rio d ic Ta b le of t he Ele m e nt s 1A 1 8A 18 1 H 1 .0 1 2A 2 3A 13 4A 14 5A 15 6A 16 7A 17 2 He 4 .0 0 3 Li 6 .9 4 4 Be 9 .0 1 5 B 1 0 .8 6 C 1 2 .0 7 N 1 4 .0 8 O 1 6 .0 9 F 1 9 .0 11 Na 2 3 .0 12 Mg 2 4 .3 3B 3 4B 4 5B 5 6B 6 7B 7 8 9 10 1B 11 2B 12 14 Si 2 8 .1 15 P 3 1 .0 16 S 3 2 .1 17 Cl 3 5 .5 19 K 3 9 .1 20 Ca 4 0 .1 21 Sc 4 5 .0 22 Ti 4 7 .9 23 V 5 0 .9 24 Cr 5 2 .0 25 Mn 5 4 .9 26 Fe 5 5 .8 27 Co 5 8 .9 28 Ni 5 8 .7 29 Cu 6 3 .5 30 Zn 6 5 .4 13 Al 2 7 .0 31 Ga 6 9 .7 10 Ne 2 0 .2 18 Ar 3 9 .9 32 Ge 7 2 .6 33 As 7 4 .9 34 Se 7 9 .0 35 Br 7 9 .9 36 Kr 8 3 .8 37 Rb 8 5 .5 38 Sr 8 7 .6 39 Y 8 8 .9 40 Zr 9 1 .2 41 Nb 9 2 .9 42 Mo 9 5 .9 43 Tc (9 8 ) 44 Ru 101 45 Rh 103 46 Pd 106 47 Ag 108 48 Cd 112 49 In 115 50 Sn 119 51 Sb 122 52 Te 128 53 I 127 54 Xe 131 55 Cs 133 56 Ba 137 57 La 139 72 Hf 178 73 Ta 181 74 W 184 75 Re 186 76 Os 190 77 Ir 192 78 Pt 195 79 Au 197 80 Hg 201 81 Tl 204 82 Pb 207 83 Bi 209 87 Fr (2 2 3 ) 88 Ra 226 89 Ac 227 8B 85 86 84 At Rn Po (2 0 9 ) (2 1 0 ) ( 2 2 2 ) 104 105 106 107 108 109 Rf Ha Unh Uns Uno Une (2 6 1 ) ( 2 6 2 ) (2 6 3 ) (2 6 2 ) ( 2 6 5 ) (2 6 6 ) Lant ha nide s 58 Ce 140 59 Pr 141 Act inide s 90 Th 232 91 Pa 231 60 Nd 144 92 U 238 61 62 63 64 65 66 67 68 69 70 71 Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu (1 4 5 ) 1 5 0 152 157 159 162 165 167 169 173 175 93 94 95 96 97 98 99 100 101 102 103 Np Pu Am Cm Bk Cf Es Fm Md No Lr (2 3 7 ) (2 4 4 ) (2 4 3 ) ( 2 4 7 ) (2 4 7 ) (2 5 1 ) ( 2 5 2 ) (2 5 7 ) (2 5 8 ) (2 5 9 ) (2 6 0 ) This project is funded by a grant awarded under the President’s Community Based Job Training Grant as implemented by the U.S. Department of Labor’s Employment and Training Administration (CB-15-162-06-60). NCC is an equal opportunity employer and does not discriminate on the following basis: against any individual in the United States, on the basis of race, color, religion, sex, national origin, age disability, political affiliation or belief; and against any beneficiary of programs financially assisted under Title I of the Workforce Investment Act of 1998 (WIA), on the basis of the beneficiary’s citizenship/status as a lawfully admitted immigrant authorized to work in the United States, or his or her participation in any WIA Title I-financially assisted program or activity. This product was funded by a grant awarded under the President’s High Growth Job Training Initiative, as implemented by the U.S. Department of Labor’s Employment & Training Administration. The information contained in this product was created by a grantee organization and does not necessarily reflect the official position of the U.S. Department of Labor. All references to nongovernmental companies or organizations, their services, products, or resources are offered for informational purposes and should not be construed as an endorsement by the Department of Labor. This product is copyrighted by the institution that created it and is intended for individual organizational, non-commercial use only.