Chemistry Free Response Practice Unit 2
advertisement

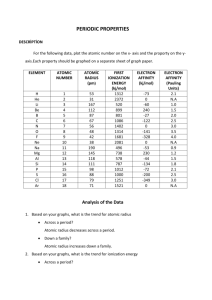
Chemistry Free Response Practice Unit 2 Credit is given for correct process, not correct answer alone. If you need the answer from an earlier part to complete a later part, but were unable to get the earlier answer, then make up an answer so that you can continue the problem. Write neatly in pencil. Erase mistakes. Use complete sentences when explaining answers and show work when solving problems (write the formula, substitute numbers with units, and box the answer—with units). 2 points each 1. Consider the elements silicon, Si (atomic number 14), phosphorus, P (atomic number 15) and sulfur, S (atomic number 16). a. Atomic radius (1) Describe core charge (Zeff) and explain how core charge affects atomic radius. Core charge = number of protons minus the number of core electrons. A greater core charge attracts the valence electrons with greater force resulting in a smaller radius. 𝑸 𝑸 Coulomb’s Law 𝑭 ≈ 𝟏 𝟐 shows that when the magnitude of the charges increases the force between the nucleus and the 𝒅 valence e- increases, causing the atomic radius to decrease. (2) Using your answer from part (1), arrange element Si, P or S from smallest atomic radius to largest. smallest middle largest S P Si b. Ionic radius (1) State one key similarity and one key difference for Si4-, P3- and S2- that affect the ionic radius. 4Si , P3- and S2- have the same number of electrons. Si4- has 14 protons, P3- has 15 protons, and S2- has 16 protons, Zeff increases. Coulombs law shows that the element with the higher Zeff, has the smaller ionic radius. (2) Using your answer from part (1), arrange element Si, P or S from smallest ionic radius to largest. smallest middle largest S2P3Si4c. First ionization energy. (1) Define first ionization energy, explain how atomic radius affects first ionization energy and what the general trend is for a period. First ionization energy is the energy needed to remove an electron from the atom. The attraction between valence electrons and core is greater for electrons closer to the core, therefore first ionization energy generally increases from left 𝑸 𝑸 to right. Coulomb’s Law 𝑬 ≈ 𝟏 𝟐 shows that when the magnitude of the charges increases the force between the nucleus 𝒅 and the valence e- increases, causing the IE to increase. (2) Draw an abbreviated orbital diagrams for Si, P and S and explain why there is an exception (anomaly) to the general trend between first ionization energy and atomic radius for these elements. Si P S [Ne] 3s() 3p()( )( ) [Ne] 3s() 3p()( )() [Ne] 3s() 3p()( )() The electron-electron repulsion raises the energy of the electrons in the fully occupied orbital, which reduces the energy needed to remove the electron from S compared to P. Yes, the half-filled p orbitals of P are stable. (3) Using your answer from parts (1) and (2), arrange element Si and P and S from lowest first ionization energy to highest. Lowest middle highest Si S P d. Electron affinity. (1) Define electron affinity, explain how atomic radius affects electron affinity and what the general trend is for a period. Electron affinity measures the change in energy when an electron is added to a neutral atom. The attraction between added electron and core is greater when the electron is closer to the core, therefore electron affinity generally becomes more negative from left to right. (2) Explain why there is an exception (anomaly) to the general trend between electron affinity and atomic radius for these elements. The electron-electron repulsion raises the energy of the electron that enters a half-filled orbital, which raises the energy state of the incoming electron, which reduces the energy released when the electron approaches the nucleus in P compared to Si. (3) Using your answer from parts (1) and (2), arrange element Si and P and S from lowest (most negative) electron affinity to highest (most positive). Lowest middle highest S Si P