Cubic Equations of State: Chemical Engineering Problems
advertisement

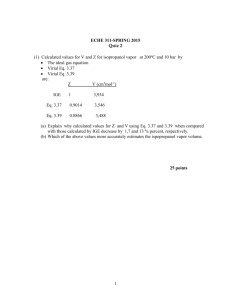
ECHE 311/ SPRING 2015 Problem Set #3 Cubic Equations of State 1. Calculate Z and V for ethylene at 25oC and 12 bar by the following equations: a) The truncated virial equation : Z= PV B C 1 RT V V2 with the following experimental values of virial coefficients: B=-140 cm3 mol-1 C=7,200 cm6 mol-2 b) The truncated virial equation Z= PV BP 1 RT RT with a value of Bo and B1 from the generalized Pitzer correlation: P P Z=1+ B o r B1 r Tr Tr c) The Redlich/Kwong equation, with the following estimates of a and b: 0.4278 R 2Tc2 0.08664 RTc a ; b Pc Pc 2. Determine Z and V for steam at 250oC and 1,800 kPa by the following: a) The truncated virial equation PV B C 1 Z= RT V V2 with the following experimental values of virial coefficients: B=-152.5 cm3 mol-1 C=-5,800 cm6 mol-2 b) The truncated virial equation: Z= PV BP 1 RT RT 1 with a value of B from the generalized Pitzer correlations: P P Z=1+ B o r B1 r Tr Tr c) The steam tables. 3. Equation Z 1 B ' P C ' P 2 D' P 3 when truncated to four terms accurately represents the volumetric data for methane gas at 0oC with B=-53.4 cm3 mol-1 C=2,620 cm6 mol-2 D= 5,000 cm9 mol-3 Plot Z vs P for methane at 0oC from 0 to 200 bar. 4. Calculate the molar volume of saturated liquid and the molar volume of saturated vapor by the Redlich/Kwong equation for propane at 40oC where Psat =13.71 bar. Compare the results with a suitable generalized correlations. 0.4278 R 2Tc2 a Pc b 0.08664 RTc Pc 5. To a good approximation, what is the molar volume of ethanol vapor at 480oC . How does this result compare with the ideal-gas value. Hint: Use the generalized virial-coefficient correlation to estimate the volume. The ideal gas equation gives a volume that is slightly larger than the volume using the generalized correlation. The percentage difference between the two values is 5.6%. 6. A 30 m3 tank contains 14 m3 of liquid n-butane in equilibrium with its vapor at 25oC. Estimate the mass of n-butane vapor in the tank. The vapor pressure of n-butane at the given temperature is 2.43 bar. Hint: Find the molar volume (Vg) using the Redlich/Kwong equation; ng 16 ; Since the liquid takes up 14 m3, the vapor will take up the rest of the available Vg volume (16 m3). The mass, mg n g M w 7. A 0.35 m5 vessel is used to store liquid propane at its vapor pressure. Safety considerations dictate that at a temperature of 320oK the liquid must occupy no more than 80% of the total volume of the vessel. For these conditions, determine the mass of vapor and the mass of liquid in the vessel. At 320oK the vapor pressure of propane is 16.0 bar. Hint: First find the molar volume (Vmg) using the Redlich/Kwong equation Next, find the molar liquid volume (Vml) using Redlich/Kwong equation Now find the mass of the liquid and vapor. 2 8. What is the pressure in a 0.5 m3 vessel when it is charged with 10 kg of carbon dioxide at 30oC. Hint: Use the Redlich/Kwong equation to calculate the pressure 3