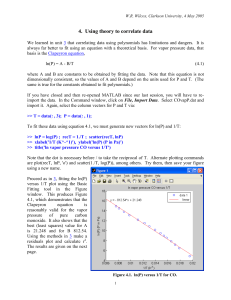
Document
advertisement

Physical equilibria:
pure substances
자연과학대학 화학과
박영동 교수
Physical equilibria: pure
substances
5.1 The thermodynamics of transition
5.1.1 The condition of stability
5.1.2 The variation of Gibbs energy with pressure
5.1.3 The variation of Gibbs energy with temperature
5.2 Phase diagrams
5.2.4 Phase boundaries
5.2.5 The location of phase boundaries
5.2.6 Characteristic points
5.2.7 The phase rule
5.2.8 Phase diagrams of typical materials
5.2.9 The molecular structure of liquids
The condition of stability
phase 2
n2
When an amount dn of the substance changes from
phase 1 with Gm(1) to phase 2 with Gm(2),
Gi = n1Gm(1) +n2Gm(2)
n1
ΔG < 0 to be spontaneous
phase 1
if Gm(2) > Gm(1), dn < 0; 2→1
ΔG = {Gm(2) − Gm(1)}dn
phase 2
if Gm(2) < Gm(1), dn > 0; 1→2
if Gm(2) = Gm(1), at equilibrium
n2 + dn
n1 - dn
phase 1
Gf = (n1 - dn)Gm(1) +(n2+dn) Gm(2)
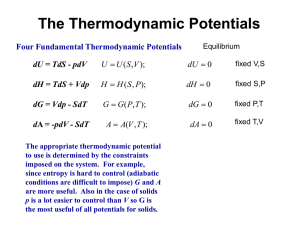
Pressure dependence of G
G = H – TS
dG = dH – TdS – SdT = Vdp - SdT
𝜕𝐺
( ) =
𝜕𝑝 𝑇
V
For liquid or solid, ΔG = VΔp
For vapor, ΔG = ∫Vdp
= nRT ∫(1/p)dp
=nRT ln(pf/pi)
ΔGm = RT ln(pf/pi)
ch05f01
Standard Gibbs Energy, ΔG⦵(p)
(
ch05f02
𝜕𝐺
) =
𝜕𝑝 𝑇
V
Calculate the Vapor pressure increase of water, when the pressure
is increased by 10 bar (Δp = 1.0 × 10 6 Pa) at 25°C.
water: density 0.997 g cm-3 at 25°C , molar volume 18.1 cm3 mol-1.
vapor, pi
Gm,i(g)
vapor, pf
Gm,f(g)
Gm,i(l)
water, p1 = 1 bar
Gm,f(l)
water, p2= 11 bar
Temperature dependence of G
G = H – TS
dG = dH – TdS – SdT = Vdp - SdT
𝜕𝐺
( 𝜕𝑝 ) = -S
𝑇
For liquid or solid,
ΔGm = -Sm ΔT
1. Sm > 0, so G will decrease as T
increases.
2. Sm(s) < Sm(l) <<Sm(g)
ch05f03
Phase Diagram
E D
H
G
C
B
A
F
ch05f05
H
G
F
Vapor pressure
ch05f06
Vapor pressure and Temperature
ch05f07
Cooling and Thermal Analysis
ch05f05
The location of phase boundaries and
Clapeyron equation
dGm(1) = Vm(1)dp − Sm(1)dT
dGm(2) = Vm(2)dp − Sm(2)dT
dGm(1) = dGm(2),
ch05f09
pvap(T ) and Clausius–Clapeyron
equation
∆𝑣𝑎𝑝 𝐻
∆(ln 𝑝) =
× ∆𝑇
𝑅𝑇 2
∆𝑣𝑎𝑝 𝐻 ∆𝑣𝑎𝑝 𝐻
ln 𝑝 = ln 𝑝′ +
−
′
𝑅𝑇
𝑅𝑇
𝐵
log 𝑝 = 𝐴 −
𝑇
The significant points of a phase
diagram
ch05f12
(a) Use the Clapeyron equation to estimate the slope of the solid–liquid
phase boundary of water given the enthalpy of fusion is 6.008 kJ mol−1 and
the densities of ice and water at 0°C are 0.916 71 and 0.999 84 g cm−3,
respectively. Hint: Express the entropy of fusion in terms of the enthalpy of
fusion and the melting point of ice.
(b) Estimate the pressure required to lower the melting point of ice by 1°C.
Usual and Unusual Substances
ch05f13
The phase rule, F=C-P+2
ch05f14
Water - phase diagram
ch05f15
Water
ch05f16
carbon dioxide
ch05f18
helium-4
superfluid
flows without viscosity
ch05f19
열역학 제1법칙은 많은 사실에 적용된다. 전압이 1.2V 인 어떤 건전지가 있
다. 이 전지가 1A의 전류로 1시간 동안 소형 모터를 작동하는데 사용되었
다.
a. 이 건전지의 일을 계산해 보시오.
b. 이 건전지의 내부에너지 변화를 계산해 보시오.
다음과 같은 관계가 Cp와 Cv 사이에 성립한다.
이는 또한 다음과 같이 표현할 수도 있다.
이 사실을 이용하여 van der Waals 기체에 대하여 다음 사실을 밝
히고, 이 값을 CO2 기체에 대하여 25℃, 1기압에서 계산해 보시오.