Quiz-2
advertisement
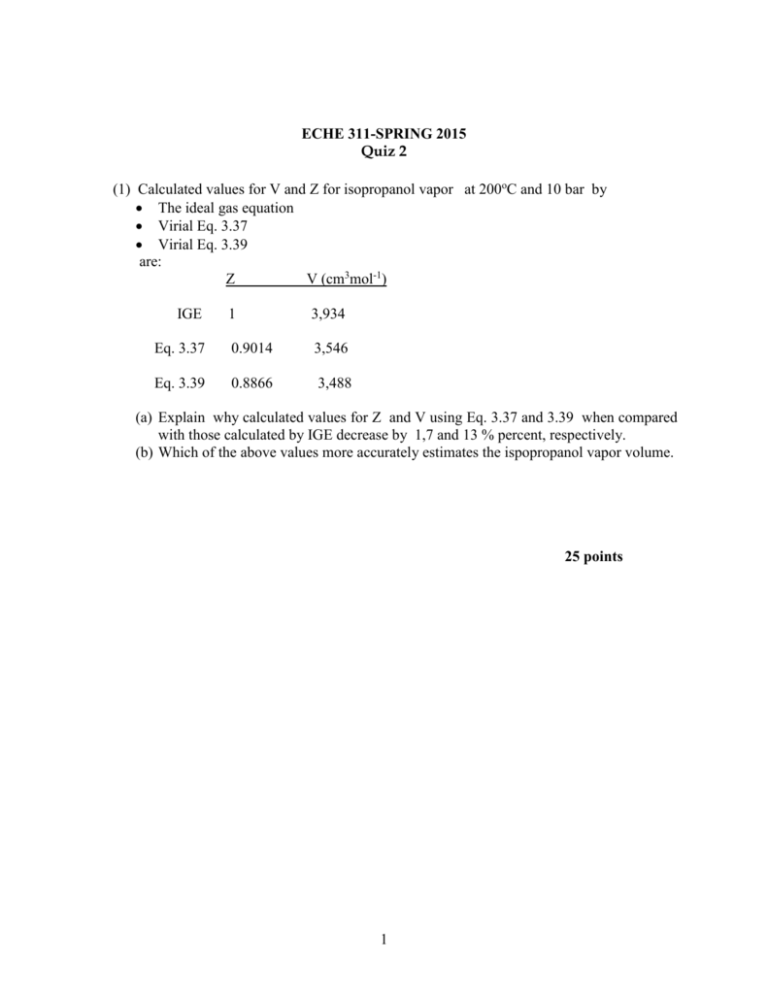
ECHE 311-SPRING 2015 Quiz 2 (1) Calculated values for V and Z for isopropanol vapor at 200oC and 10 bar by The ideal gas equation Virial Eq. 3.37 Virial Eq. 3.39 are: Z V (cm3mol-1) IGE 1 3,934 Eq. 3.37 0.9014 3,546 Eq. 3.39 0.8866 3,488 (a) Explain why calculated values for Z and V using Eq. 3.37 and 3.39 when compared with those calculated by IGE decrease by 1,7 and 13 % percent, respectively. (b) Which of the above values more accurately estimates the ispopropanol vapor volume. 25 points 1 (2) The acentric factor is defined at Tr=0.7 is: 1.0 log( Prsat )Tr Explain when Zo in Pitzer correlation Z Z o wZ 1 becomes identical to Z. 25 points 2 3. Explain the compressibility-factor graph for methane. 25 points 3 (4) Identify which of the relationships given bellow is Lee /Kesler correlation at constant Tr? (a) ( Z) vs Pr (b) (Zo) vs Pr (c) (Z1) vs Pr (d) (ZoZ1) vs Pr 25 points 4











