Powerpoint
advertisement
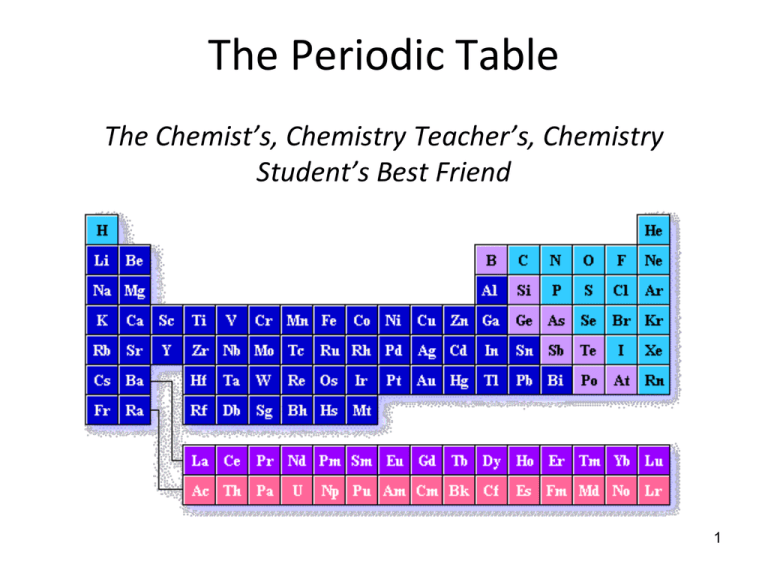
The Periodic Table The Chemist’s, Chemistry Teacher’s, Chemistry Student’s Best Friend 1 Poetry Assignment • Pick an element • Write a poem about it without using its name • Read the poem to the class and see if we can figure it out! What is the Periodic Table? • The periodic table is an organized arrangement of the elements • Over time, and as more elements were discovered, scientists noticed repeating patterns of properties among elements • They tried to organize the elements by these properties 3 JOHANN DOBEREINER (1829) The Model of Triads • Classified some elements into groups of three, which he called triads • The elements in a triad had similar chemical properties and orderly physical properties • One element in each triad had properties intermediate of the other two elements • Examples: – Cl, Br, I – Ca, Sr, Ba 4 JOHN NEWLANDS (1863) The Law of Octaves • Arranged elements in order of increasing atomic mass • Noticed that certain properties repeated every eighth element • Elements should be arranged in “octaves” (like in music) • His law of octaves failed beyond the element calcium • His claim was dismissed out of hand • But this idea of “repeating 8’s” will re-surface! 5 DMITRI MENDELEEV (late 1800’s) • He is credited with “inventing” the periodic table, although other folks were also doing similar work around the same time • He arranged the known elements by increasing atomic mass • When arranged this way, he noticed that properties did repeat • He left blank spaces to keep elements properly aligned by their properties • He felt that the blank spaces would someday be occupied by elements that had not yet been discovered • He predicted the properties of those elements – his predictions were remarkably accurate 6 But Mendeleev’s Periodic Table Was Not Perfect … • In spite of his great achievement, problems arose when new elements were discovered and more accurate atomic weights determined • Examples: – Ar and K – Co and Ni – Te and I – Th and Pa 7 HENRY G.J. MOSELEY (1913) • The old arrangement was not perfect – some elements just didn’t fit • Improved the periodic table by arranging elements by atomic number instead of atomic mass • For the most part, atomic masses also increase, although there are some exceptions (examples are cobalt and nickel) • The new arrangement corrected the problems from the old version • Current arrangement of periodic table is by atomic number 8 Sad Ending … • Henry Moseley was killed by a sniper in 1915 during World War I (Gallipoli, Turkey) • Because of this, the British government changed its policy and did not allow “prominent and promising scientists” to enlist for combat duty in the armed forces during World War II 9 The Periodic Law: When elements are arranged in order of increasing atomic number, there is a periodic repetition of their physical and chemical properties 10 Periodic or cyclical events • Can you think of some examples of phenomena or events that are periodic or cyclical? • • • • • Seasons Weeks Phases of the moon Octaves in music Waves Let’s pretend we are Mendeleev’s partner trying to figure out the periodic table! EXAMPLE PERIODIC TABLE ENTRY Note that the atomic number is a whole # and the atomic mass is a decimal # 12 NOMENCLATURE OF ELEMENTS – CHEMICAL SYMBOLS • Could be the 1st letter of the element – H=hydrogen • May need the 2nd or another letter of the element – C=carbon, so what about cobalt or calcium? Needed 2nd letter. What about chlorine? 6 elements have “C” as 1st letter! • Some derived from Latin name – Au=gold (aurum = shining dawn) • Some named to honor scientists (Cm=curium for Marie Curie) • Some named for planets (U=uranium, Np=neptunium, Pu=plutonium) 13 NOMENCLATURE OF ELEMENTS – CHEMICAL SYMBOLS • Some named for places (Eu = Europium, Ytterby in Sweden has four elements named for it: Tb = terbium, Y = yttrium, Er = erbium, Yb = ytterbium) • Some named for properties (Cs = cesium because it imparts a blue color to a flame, caesius = sky blue) • Some named using rules established by an international committee • First letter is ALWAYS capitalized; second letter is ALWAYS lower case 14 Trivia Question: What is the only letter of the alphabet not to appear in the periodic table? 15 Trivia Question: What is the only letter of the alphabet not to appear in the periodic table? J 16 There are many ways to “slice up” the periodic table Let’s look at some of them … 17 One way to examine the periodic table – Break it up element type What’s up with the heavy black line? 18 Metals, Non-Metals, Metalloids • That heavy stair-step line separates the metals from the non-metals • The elements that straddle the line are called “metalloids” 19 Properties of Metals • Metals are good conductors of heat and electricity • Metals are shiny • Metals are ductile (can be stretched into thin wires) • Metals are malleable (can be pounded into thin sheets) • A chemical property of metal is its reaction with water, which results in corrosion 20 Properties of Non-Metals • Non-metals are poor conductors of heat and electricity • Non-metals are not ductile or malleable • Solid non-metals are brittle and break easily • They are dull (non-lustrous); they do not reflect light • Some non-metals are gases (O, N, Cl); some are brittle solids (S); one is a orange liquid (Br) 21 Properties of Metalloids • Metalloids (metal-like) have properties of both metals and nonmetals • Some of the metalloids are semiconductors. This means that they can carry an electrical charge under special conditions. This property makes metalloids useful in computers and calculators • They are solids that can be shiny or dull • They conduct heat and electricity better than non-metals but not as well as metals • They are ductile and malleable 22 Another Way to Divvy Up the Periodic Table What about rows and columns? 23 Rows on the Periodic Table • Each horizontal row of the periodic table is called a period – There are 7 periods – The elements in a period are not alike in properties – The properties change greatly across even given row – The first element in a period is always an extremely active solid; the last element in a period is always an inactive gas 24 Columns on the Periodic Table • The vertical columns of elements on the periodic table are called groups or families – The elements in any group/family of the periodic table have similar, but not identical, physical and chemical properties – Groups/Families have names in addition to numbers – Groups/Families are numbered from 1-18 (left-right) or I-IIA, B, III-VIIIA (left-right) – The elements in each column have the same number of electrons in the outermost energy level or shell (one to eight → remember John Newland’s “law of octaves”?) 25 Elements Investigation Lab • Now’s it is your turn to get to see some of these elements up close and personal • You and a partner will visit a representative sample of elements and record physical information about each Let’s Explore Yet Another Way to Divvy Up the Periodic Table - by Families! 27 GROUP 1 - ALKALI METALS •Group 1 •very reactive metals that do not occur freely in nature •malleable, ductile, good conductors of heat and electricity. •can explode if they are exposed to water Alkali Metals Alkali Metals • The alkali metals form compounds that are similar to each other. • Alkali metals each have one valence electron • This electron is removed when alkali metals react. • The easier it is to remove an electron, the more reactive the atom is. • TREND: Reactivity of alkali metals increase as you go down the group. ALKALINE EARTH METALS •Group 2 •metals •very reactive •not found free in nature Alkaline Earth Metals • Alkaline Earth metals each have TWO valence electrons • These electrons are removed when alkaline earth metals react. • The easier it is to remove an electron, the more reactive the atom is. • TREND: Reactivity of alkali metals increase as you go down the group. TRANSITION METALS •Group 3-12 •ductile and malleable, and conduct electricity and heat •iron, cobalt, and nickel, are the only elements known to produce a magnetic field. RARE EARTH ELEMENTS •many are man-made From www.science-class.net Boron, Carbon, Nitrogen & Oxygen Families Named after the head of each family These elements are usually referred as either metals, metalloids or non metals rather than their family names P-block METALS •are ductile and malleable •are solid, have a high density, HALOGENS – Group 17 •Group 17 •"halogen" means "salt-former" and compounds containing halogens are called "salts" •exist in all three states of matter Halogens – group 17 • Fluorine is the most reactive of the halogens because its outer energy level is closest to the nucleus. • The reactivity of the halogens decreases down the group as the outer energy levels of each element’s atoms get farther from the nucleus. NOBLE GASES •Group 18 •do not form compounds easily •Happy/Inert Elements (Full outer shells) From www.science-class.net Noble gases – group 18 • Neon and the elements below it in Group 18 have eight electrons in their outer energy levels. • Their energy levels are stable, so they do not combine easily with other elements. Noble gases • At one time these elements were thought to be completely unreactive, and therefore became known as the inert gases. • When chemists learned that some of these gases can react, their name was changed to noble gases. • They are still the most stable element group. Coloring Activity • Okay…need a way to organize all of this information I just gave you? • You and a partner will now work on the Getting to know the Periodic Table Coloring Activity